


| Pair Name | Phenethyl isothiocyanate, Dibenzoylmethane | ||
| Phytochemical Name | Phenethyl isothiocyanate (PubChem CID: 16741 ) | ||
| Anticancer drug Name | Dibenzoylmethane (PubChem CID: 8433 ) | ||
| Structure of Phytochemical |
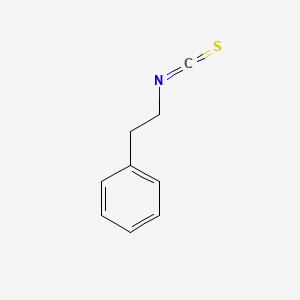
|
Download
2D
MOL
3D
MOL
|
|
| Structure of Anticancer Drug |

|
Download
2D
MOL
3D
MOL
|
|
| Pair Name | Phenethyl isothiocyanate, Dibenzoylmethane | |||
| Disease Info | [ICD-11: 2C82.0] | Prostate cancer | Investigative | |
| Biological Phenomena | Induction-->Apoptosis | |||
| Gene Regulation | Down-regulation | Phosphorylation | AKT1 | hsa207 |
| Down-regulation | Expression | BIRC5 | hsa332 | |
| Down-regulation | Activity | CDH13 | hsa1012 | |
| In Vitro Model | PC-3 | Prostate carcinoma | Homo sapiens (Human) | CVCL_0035 |
| DU145 | Prostate carcinoma | Homo sapiens (Human) | CVCL_0105 | |
| VCaP | Prostate carcinoma | Homo sapiens (Human) | CVCL_2235 | |
| In Vivo Model | For a xenograft model, 2×10⁶ VCaP cells per 0.1 ml suspended in a mixture of Matrigel and RPMI 1640 medium (1 : 1) were injected subcutaneously into the back of the mice. | |||
| Result | Our results indicate that administration of DBM and PEITC in combination may be an effective strategy for inhibiting/delaying the progression of prostate cancer to androgen independence. | |||
| No. | Title | Href |
|---|---|---|
| 1 | Phenethyl isothiocyanate in combination with dibenzoylmethane inhibits the androgen-independent growth of prostate cancer cells. Food Funct. 2018 Apr 25;9(4):2398-2408. doi: 10.1039/c7fo01983a. | Click |
