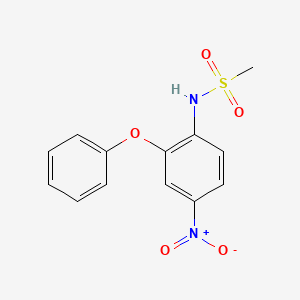
nimesulide, 51803-78-2, N-(4-Nitro-2-phenoxyphenyl)methanesulfonamide, Mesulid, Flogovital, Sulidene, Nimed, 4-NITRO-2-PHENOXYMETHANESULFONANILIDE, R-805, Methanesulfonamide, N-(4-nitro-2-phenoxyphenyl)-, Nimesulidum, Nimesulida, R 805, 4'-Nitro-2'-phenoxymethanesulfonanilide, Nisulid, 4-Nitro-2-phenoxy-methanesulfonanilide, EINECS 257-431-4, UNII-V4TKW1454M, 4'-Nitro-2'-phenoxymethansulfonanilid, NSC-758412, BRN 2421175, CCRIS 8225, V4TKW1454M, DTXSID3037250, CHEBI:44445, Methanesulfonanilide, 4'-nitro-2'-phenoxy-, CHEMBL56367, MLS000069680, DTXCID1017250, MFCD00079470, NSC 758412, NCGC00015725-02, SMR000058484, CAS-51803-78-2, NIMESULIDE (MART.), NIMESULIDE [MART.], NIMESULIDE (EP IMPURITY), NIMESULIDE [EP IMPURITY], NIMESULIDE (EP MONOGRAPH), NIMESULIDE [EP MONOGRAPH], Antifloxil, Guaxan, Nimesulidum [INN-Latin], Nimesulida [INN-Spanish], NIM, Nimesulide [INN:BAN], SR-01000000218, Aldoron, Nimedex, Orthobid, Nise Gel, Nimesulide,(S), Prestwick_618, Spectrum_001577, NIMESULIDE [MI], Nimesulide (JAN/INN), NIMESULIDE [INN], NIMESULIDE [JAN], Opera_ID_1247, Prestwick0_000194, Prestwick1_000194, Prestwick2_000194, Prestwick3_000194, Spectrum2_001541, Spectrum3_001576, Spectrum4_000178, Spectrum5_000964, Lopac-N-1016, N-(4-nitro-2-phenoxy-phenyl)methanesulfonamide, NIMESULIDE [WHO-DD], Lopac0_000855, SCHEMBL24882, BSPBio_000147, BSPBio_001103, BSPBio_003112, KBioGR_000443, KBioGR_000695, KBioSS_000443, KBioSS_002057, MLS001148268, DivK1c_000693, SPECTRUM1503231, SPBio_001382, SPBio_002068, N-(4-nitro-2-phenoxyphenyl), BPBio1_000163, GTPL7401, NIM-03, BCBcMAP01_000034, HMS502C15, KBio1_000693, KBio2_000443, KBio2_002057, KBio2_003011, KBio2_004625, KBio2_005579, KBio2_007193, KBio3_000825, KBio3_000826, KBio3_002612, M01AX17, Nimesulide for peak identification, NINDS_000693, Bio2_000382, Bio2_000862, HMS1362G05, HMS1568H09, HMS1792G05, HMS1922K17, HMS1990G05, HMS2089B14, HMS2095H09, HMS2234K19, HMS3262L11, HMS3269G17, HMS3371J19, HMS3403G05, HMS3414P09, HMS3649A04, HMS3655D13, HMS3678P07, HMS3712H09, HMS3884C22, Pharmakon1600-01503231, BCP10076, HY-B0363, Tox21_110207, Tox21_301850, Tox21_500855, BDBM50056999, CCG-39319, EI-287, NSC758412, s2040, STL018679, AKOS015897356, Tox21_110207_1, AC-4524, DB04743, KS-1277, LP00855, SDCCGSBI-0050831.P004, IDI1_000693, IDI1_002137, NCGC00015725-01, NCGC00015725-03, NCGC00015725-04, NCGC00015725-05, NCGC00015725-06, NCGC00015725-07, NCGC00015725-08, NCGC00015725-09, NCGC00015725-10, NCGC00015725-11, NCGC00015725-12, NCGC00015725-13, NCGC00015725-15, NCGC00015725-16, NCGC00015725-29, NCGC00021842-03, NCGC00021842-04, NCGC00021842-05, NCGC00021842-06, NCGC00021842-07, NCGC00021842-08, NCGC00255661-01, NCGC00261540-01, BN166246, Nimesulide 100 microg/mL in Acetonitrile, R805, N-(4-Nitro-2-Phenoxy)Methanesulfonanilide, SBI-0050831.P003, DB-052029, AB00052332, EU-0100855, FT-0630650, N0984, NS00006725, SW196785-3, D01049, D70376, M02164, N 1016, Q20994, AB00052332-16, AB00052332_17, AB00052332_18, EN300-7388636, N-[4-nitro-2-(phenoxy)phenyl]methanesulfonamide, A828786, SR-01000000218-2, SR-01000000218-6, SR-01000000218-7, W-105866, BRD-K76775527-001-06-2, BRD-K76775527-001-18-7, SR-01000000218-11, Nimesulide, European Pharmacopoeia (EP) Reference Standard, Nimesulide, Pharmaceutical Secondary Standard; Certified Reference Material, Nimesulide for peak identification, European Pharmacopoeia (EP) Reference Standard, 1364966-82-4