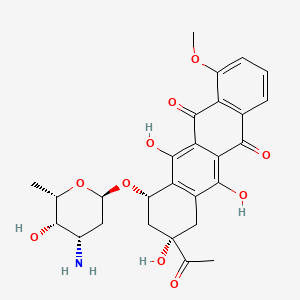
daunorubicin, Daunomycin, 20830-81-3, Acetyladriamycin, Leukaemomycin C, Rubidomycin, Cerubidine, (+)-Daunomycin, DaunoXome, Daunorubicinum, Daunorubicine, Cerubidin, RP 13057, Rubomycin C, FI 6339, Daunorubicin (INN), NSC-82151, Daunarubicinum, Daunorrubicina, DaunoXome (TN), Daunamycin, FI-6339, FI6339, ZS7284E0ZP, (7S,9S)-9-acetyl-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione, (8S-cis)-8-Acetyl-10-((3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyrannosyl)oxy)-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-5,12-napthacenedione, DTXSID7022883, CHEBI:41977, RP-13057, 5,12-Naphthacenedione,8-acetyl-10-[(3-amino-2,3,6-trideoxy-a-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-, (8S,10S)-, NCGC00024246-05, Anthracycline, Anthracyline, NDC-0082-4155, DAUNORUBICIN [INN], Daunorubicinum [INN-Latin], DTXCID402883, MLS000069508, Daunorubicin [INN:BAN], NSC-83142, RCRA waste no. U059, (1S,3S)-3-acetyl-3,5,12-trihydroxy-10-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranoside, (8S,10S)-8-acetyl-10-(((2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-6,8,11-trihydroxy-1-methoxy-7,8,9,10-tetrahydrotetracene-5,12-dione, (8S,10S)-8-acetyl-10-{[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy}-6,8,11-trihydroxy-1-methoxy-5,7,8,9,10,12-hexahydrotetracene-5,12-dione, CAS-20830-81-3, NSC82151, SMR000058559, CCRIS 914, SR-01000000033, SR-05000001600, HSDB 5095, NCI-C04693, EINECS 244-069-7, NSC 83142, VS-103, BRN 1445583, Tocris-1467, Daunorubicin(Daunomycin), AI3-52942, Prestwick3_000487, DAUNOMYCIN [IARC], DAUNORUBICIN [MI], CHEMBL178, DAUNORUBICIN [HSDB], SCHEMBL3041, DAUNORUBICIN [VANDF], EPIRUBICIN IMPURITY D, UNII-ZS7284E0ZP, BSPBio_000353, DAUNORUBICIN [MART.], 5,12-Naphthacenedione, 8-acetyl-10-((3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-, (8S-cis)-, cid_62770, DAUNORUBICIN [WHO-DD], BPBio1_000389, GTPL7063, BDBM32017, EX-A1337A, Valrubicin impurity, daunorubicin, Daunomycin;RP 13057;Rubidomycin, DAUNORUBICIN [ORANGE BOOK], HMS2089H04, HMS2091K06, Pharmakon1600-01500223, VYXEOS COMPONENT DAUNORUBICIN, Tox21_110896, BDBM50368352, GR-318, HY-13062A, LMPK13050002, MFCD00866340, NSC756717, Tox21_110896_1, CCG-212559, CS-2004, DB00694, NSC-756717, NCGC00024246-06, NCGC00024246-07, NCGC00024246-09, NCGC00024246-10, NCGC00024246-12, NCGC00024246-15, NCGC00024246-18, NCGC00025173-01, (1S,3S)-3-acetyl-3,5,12-trihydroxy-10-(methyloxy)-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranoside, (1S,3S)-3-acetyl-3,5,12-trihydroxy-10-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-a-L-lyxo-hexopyranoside, (7S,9R)-9-Acetyl-7-[(2S,4S,5S,6S)-4-amino-5-hydroxy-6-methyl-oxan-2-yl]oxy-6,9,11-trihydroxy-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione, (7S,9S)-9-acetyl-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyl-tetrahydropyran-2-yl]oxy-6,9,11-trihydroxy-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione, 5,12-Naphthacenedione, 8-acetyl-10-((3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-, (8S,10S)-, SBI-0206677.P002, AB00514669, C01907, D07776, Epirubicin hydrochloride impurity, daunorubicin-, AB00514669-09, AB01644616_09, AB01644616_10, EN300-7479232, A814957, Q411659, SR-01000000033-4, SR-05000001600-1, SR-05000001600-2, BRD-K43389675-001-01-3, BRD-K43389675-003-02-7, BRD-K43389675-003-03-5, BRD-K43389675-003-20-9, EPIRUBICIN HYDROCHLORIDE IMPURITY D [EP IMPURITY], VALRUBICIN IMPURITY, DAUNORUBICIN [USP IMPURITY], DOXORUBICIN HYDROCHLORIDE IMPURITY A [EP IMPURITY], EPIRUBICIN HYDROCHLORIDE IMPURITY, DAUNORUBICIN- [USP IMPURITY], (1S,3S)-3-ACETYL-1,2,3,4,6,11-HEXAHYDRO-3,5,12-TRIHYDROXY-10-METHOXY-6,11-DIOXO-1-NAPHTHACENYL 3-AMINO-2,3,6-TRIDEOXY-.ALPHA.-L-LYXO-HEXOPYRANOSIDE, (7S,9S)-7-[(2R,4S,5S,6S)-4-azanyl-6-methyl-5-oxidanyl-oxan-2-yl]oxy-9-ethanoyl-4-methoxy-6,9,11-tris(oxidanyl)-8,10-dihydro-7H-tetracene-5,12-dione;hydrochloride, (7S,9S)-9-acetyl-7-(4-amino-5-hydroxy-6-methyl-tetrahydropyran-2-yl)oxy-6,9,11-trihydroxy-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione chloride, (7S,9S)-9-acetyl-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyl-tetrahydropyran-2-yl]oxy-6,9,11-trihydroxy-4-methoxy-8,10-dihydro-7H-tetracene-5,12-quinone;hydrochloride, (7S,9S)-9-acetyl-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione;hydrochloride, (7S,9S)-9-acetyl-7-[[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyl-2-oxanyl]oxy]-6,9,11-trihydroxy-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione;hydrochloride, (8S,10S)-8-ACETYL-10-((3-AMINO-2,3,6-TRIDEOXY-.ALPHA.-L-LYXO-HEXOPYRANOSYL)OXY)-6,8,11-TRIHYDROXY-1-METHOXY-7,8,9,10-TETRAHYDROTETRACENE-5,12-DIONE, (8S-cis)-8-Acetyl-10-[(3-amino-2,3,6-trideoxy-.alpha.-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro--6,8,11-trihydroxy-1-methoxy-5,12-naphthacenedione, 5,12-NAPHTHACENEDIONE, 8-ACETYL-10-((3-AMINO-2,3,6-TRIDEOXY-.ALPHA.-L-LYXO-HEXOPYRANOSYL))OXY)-7,8,9,10-TETRAHYDRO-6,8,11-TRIHYDROXY-1-METHOXY-, (8S-CIS)-