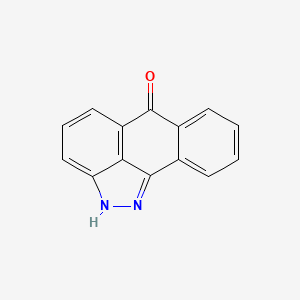
129-56-6, 1,9-Pyrazoloanthrone, SP600125, Pyrazolanthrone, Dibenzo[cd,g]indazol-6(2H)-one, Pyrazoleanthrone, SP 600125, SP-600125, Anthra[1,9-cd]pyrazol-6(2H)-one, Anthra-1,9-pyrazol-6-none, JNK Inhibitor II, ANTHRA(1,9-cd)PYRAZOL-6(2H)-ONE, C.I. 70300, 2H-Dibenzo[cd,g]indazol-6-one, NSC 75890, NSC-75890, 2h-dibenzo(cd,g)indazol-6-one, NSC75890, CHEMBL7064, MLS002693964, DTXSID2040525, CHEBI:90695, MFCD00022289, 1TW30Y2766, NCGC00015958-03, 14,15-diazatetracyclo[7.6.1.02,7.013,16]hexadeca-1(15),2,4,6,9(16),10,12-heptaen-8-one, SMR000015440, SR-01000075840, EINECS 204-955-6, BRN 0746890, UNII-1TW30Y2766, 1pmv, 2zmd, Anthra[1-9-cd]pyrazol-6(2H)-one, Kinome_3844, Tocris-1496, CI 70300, BiomolKI_000068, Lopac-S-5567, BiomolKI2_000072, cid_8515, CBiol_002049, Lopac0_000473, BMK1-G8, BSPBio_001066, ChemBiol10705 Compound 4, KBioGR_000406, KBioSS_000406, JMC517015 Compound 2, MLS002153267, MLS006011577, SCHEMBL170980, anthra[1,9-cd]pyrazol-6-one, GTPL5273, Pyrazolanthrone (SP600125), CHEMBL1725279, DTXCID0020525, SCHEMBL15583517, BCBcMAP01_000053, BDBM16018, dibenz[cd,g]indazol-6(2h)-one, KBio2_000406, KBio2_002974, KBio2_005542, KBio3_000771, KBio3_000772, Bio1_000335, Bio1_000824, Bio1_001313, Bio2_000373, Bio2_000853, GLXC-03091, HMS1362F07, HMS1667K13, HMS1792F07, HMS1990F07, HMS2250C03, HMS3229I16, HMS3261O08, HMS3267P06, HMS3295M01, HMS3403F07, HMS3412F05, HMS3654P10, HMS3676F05, HMS3747M19, PS653, ALBB-024051, AMY31086, Anthra[1,9cd]pyrazol-6(2H)-one, BCP05457, EX-A1998, Tox21_110267, Tox21_500473, BDBM50024294, BDBM50433916, CCG-47500, Dibenzo[cd,g]indazol-6(2H)-one #, HB2234, HSCI1_000136, NSC755773, s1460, AKOS000115584, AKOS040751313, Anthrapyrazolone; 1,9-Pyrazoloanthrone, CCG-100672, CS-0196, DB01782, LP00473, NSC-755773, SDCCGSBI-0050458.P003, WLN: T C66651A P IV OMNJ, 1,6-dihydrodibenzo[cd,g]indazol-6-one, 2,6-dihydrodibenzo[cd,g]indazol-6-one, IDI1_002128, QTL1_000077, s10332, SMP2_000240, NCGC00015958-01, NCGC00015958-02, NCGC00015958-04, NCGC00015958-05, NCGC00015958-06, NCGC00015958-07, NCGC00015958-08, NCGC00015958-22, NCGC00025186-01, NCGC00025186-02, NCGC00025186-03, NCGC00025186-04, NCGC00025186-05, NCGC00261158-01, WLN: T C6665 1A P IV OMNJ, AC-32051, AS-14374, CAS-129-56-6, HY-12041, JNK Inhibitor II - CAS 129-56-6, SMR002530644, SY052909, DB-041928, EU-0100473, FT-0607068, NS00020981, SW218106-2, EN300-02083, Anthra[1,9-cd]pyrazol-6(2H)-one & Z-100, K00068, S 5567, SP600125, >=98% (HPLC), AB00075935-01, SP 600125 & Z-100, A888840, Anthra[1,9-cd]pyrazol-6(2H)-one;SP-600125, Q4545713, SR-01000075840-1, SR-01000075840-2, SR-01000075840-4, SR-01000075840-6, SR-01000637108-1, W-108360, BRD-K01567962-001-04-0, BRD-K01567962-001-06-5, BRD-K01567962-001-08-1, BRD-K01567962-001-22-2, Z56785477, F1414-1245, 1PMV , JNK Inhibitor II , Nsc75890 , Pyrazolanthrone, 14,15-diazatetracyclo[7.6.1.0;{2,7}.0;{13,16}]hexadeca-1(15),2(7),3,5,9(16),10,12-heptaen-8-one, 14,15-diazatetracyclo[7.6.1.0^{2,7}.0^{13,16}]hexadeca-1(15),2,4,6,9(16),10,12-heptaen-8-one