NPs Basic Information

|
Name |
3-Hydroxybenzyl alcohol
|
| Molecular Formula | C7H8O2 | |
| IUPAC Name* |
3-(hydroxymethyl)phenol
|
|
| SMILES |
C1=CC(=CC(=C1)O)CO
|
|
| InChI |
InChI=1S/C7H8O2/c8-5-6-2-1-3-7(9)4-6/h1-4,8-9H,5H2
|
|
| InChIKey |
OKVJCVWFVRATSG-UHFFFAOYSA-N
|
|
| Synonyms |
3-hydroxybenzyl alcohol; 620-24-6; 3-(Hydroxymethyl)phenol; Benzenemethanol, 3-hydroxy-; 3-Hydroxybenzenemethanol; m-Hydroxybenzyl alcohol; Benzyl alcohol, m-hydroxy-; KSD 2405; 3-Hydroxybenzylalcohol; MFCD00004643; 3-Hydroxymethyl-phenol; 3-(hydroxymethyl)-phenol; H652F6XF7Y; CHEBI:17069; NSC-60735; EINECS 210-633-6; NSC 60735; AI3-31880; 3-hydroxymethylphenol; 3-hydroxymethyl phenol; 3-(Hydroxymethy)phenol; 3-hydroxy benzyl alcohol; 3-hydroxy-phenyl-methanol; 3-(hydroxy)benzyl alcohol; 3-(Hydroxymethyl)phenol #; 3-[(Hydroxy)methyl]phenol; UNII-H652F6XF7Y; SCHEMBL96097; 3-Hydroxybenzyl alcohol, 97%; 3-Hydroxybenzyl alcohol, 99%; CHEMBL3337531; DTXSID20211035; HYDROXYBENZYL ALCOHOL, M-; ZINC388761; NSC60735; s6363; AKOS000249424; AC-10893; AS-12681; HY-78446; SY006445; DB-054044; CS-0008490; FT-0615835; H0918; EN300-55888; C03351; 620H246; A833547; W-105054; Q27102196; F0001-1652; Z335245000; [3,4,6-triacetoxy-5-(dimethylcarbamoylamino)tetrahydropyran-2-yl]methyl acetate;3-Hydroxybenzyl Alcohol; 3HY
|
|
| CAS | 620-24-6 | |
| PubChem CID | 102 | |
| ChEMBL ID | CHEMBL3337531 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 124.14 | ALogp: | 0.5 |
| HBD: | 2 | HBA: | 2 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 40.5 | Aromatic Rings: | 1 |
| Heavy Atoms: | 9 | QED Weighted: | 0.592 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.246 | MDCK Permeability: | 0.00002160 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.005 |
| Human Intestinal Absorption (HIA): | 0.016 | 20% Bioavailability (F20%): | 0.769 |
| 30% Bioavailability (F30%): | 0.311 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.091 | Plasma Protein Binding (PPB): | 26.59% |
| Volume Distribution (VD): | 1.232 | Fu: | 70.98% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.638 | CYP1A2-substrate: | 0.202 |
| CYP2C19-inhibitor: | 0.095 | CYP2C19-substrate: | 0.204 |
| CYP2C9-inhibitor: | 0.019 | CYP2C9-substrate: | 0.336 |
| CYP2D6-inhibitor: | 0.211 | CYP2D6-substrate: | 0.616 |
| CYP3A4-inhibitor: | 0.025 | CYP3A4-substrate: | 0.21 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 12.09 | Half-life (T1/2): | 0.911 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.04 | Human Hepatotoxicity (H-HT): | 0.035 |
| Drug-inuced Liver Injury (DILI): | 0.05 | AMES Toxicity: | 0.039 |
| Rat Oral Acute Toxicity: | 0.499 | Maximum Recommended Daily Dose: | 0.015 |
| Skin Sensitization: | 0.862 | Carcinogencity: | 0.085 |
| Eye Corrosion: | 0.127 | Eye Irritation: | 0.992 |
| Respiratory Toxicity: | 0.04 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000756 |  |
0.710 | D05OIS |  |
0.455 | ||
| ENC003374 | 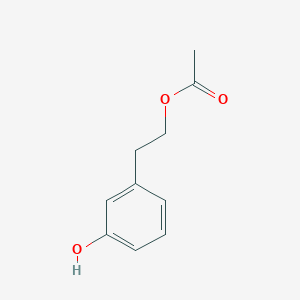 |
0.488 | D0O6IU | 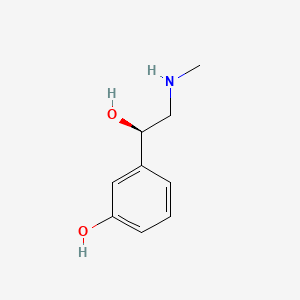 |
0.450 | ||
| ENC000014 |  |
0.455 | D04EYC | 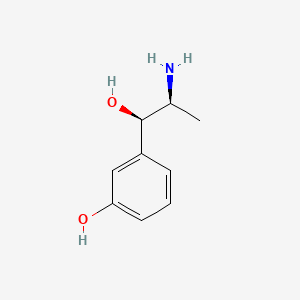 |
0.425 | ||
| ENC000985 |  |
0.444 | D0S5LH |  |
0.425 | ||
| ENC001049 |  |
0.444 | D03UOT |  |
0.343 | ||
| ENC000394 |  |
0.421 | D0K4MH |  |
0.333 | ||
| ENC000714 |  |
0.395 | D02JIS | 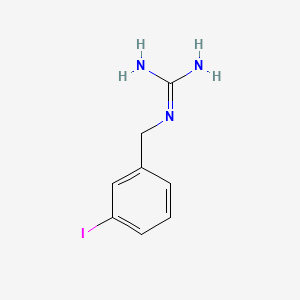 |
0.318 | ||
| ENC000350 |  |
0.395 | D0T7OW |  |
0.310 | ||
| ENC000413 |  |
0.389 | D01CRB |  |
0.304 | ||
| ENC002426 | 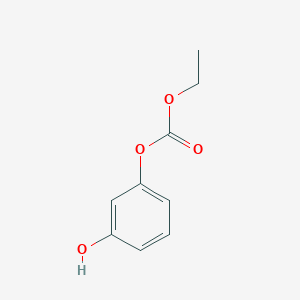 |
0.386 | D0S5YC |  |
0.304 | ||