NPs Basic Information

|
Name |
4-Methoxybenzyl formate
|
| Molecular Formula | C9H10O3 | |
| IUPAC Name* |
(4-methoxyphenyl)methyl formate
|
|
| SMILES |
COC1=CC=C(C=C1)COC=O
|
|
| InChI |
InChI=1S/C9H10O3/c1-11-9-4-2-8(3-5-9)6-12-7-10/h2-5,7H,6H2,1H3
|
|
| InChIKey |
XPDORSROGAZEGY-UHFFFAOYSA-N
|
|
| Synonyms |
4-Methoxybenzyl formate; Anisyl formate; 122-91-8; (4-methoxyphenyl)methyl formate; p-Methoxybenzyl formate; Anisyl alcohol, formate; Anisyl methanoate; Benzenemethanol, 4-methoxy-, 1-formate; p-Methoxybenzyl alcohol, formate; BENZENEMETHANOL, 4-METHOXY-, FORMATE; p-Anisyl formate; Benzyl alcohol, p-methoxy-, formate; 4-Methoxybenzenemethyl formate; FEMA No. 2101; 7N0ADO5BXI; NSC-5949; Anisyl formate (natural); Methoxybenzyl methanoate, p-; NSC 5949; EINECS 204-582-9; UNII-7N0ADO5BXI; AI3-02941; para-anisyl formate; anisyl alcohol formate; p-Methoxybenzyl methanoate; SCHEMBL6342; DSSTox_CID_27649; DSSTox_RID_82476; DSSTox_GSID_47649; ANISYL FORMATE [FCC]; ANISYL FORMATE [FHFI]; (4-methoxyphenyl)methyl ormate; CHEMBL3188181; DTXSID7047649; FEMA 2101; NSC5949; CHEBI:173752; ZINC1687335; Tox21_302535; Anisyl formate, >=90%, FCC, FG; AKOS024319071; NCGC00256666-01; AS-63839; CAS-122-91-8; Benzyl alcohol, p-methoxy-, formate (8CI); Q27268587
|
|
| CAS | 122-91-8 | |
| PubChem CID | 61054 | |
| ChEMBL ID | CHEMBL3188181 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 166.17 | ALogp: | 1.7 |
| HBD: | 0 | HBA: | 3 |
| Rotatable Bonds: | 4 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 35.5 | Aromatic Rings: | 1 |
| Heavy Atoms: | 12 | QED Weighted: | 0.64 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.329 | MDCK Permeability: | 0.00003540 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.001 |
| 30% Bioavailability (F30%): | 0.169 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.978 | Plasma Protein Binding (PPB): | 41.83% |
| Volume Distribution (VD): | 2.208 | Fu: | 42.16% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.974 | CYP1A2-substrate: | 0.881 |
| CYP2C19-inhibitor: | 0.926 | CYP2C19-substrate: | 0.713 |
| CYP2C9-inhibitor: | 0.306 | CYP2C9-substrate: | 0.814 |
| CYP2D6-inhibitor: | 0.577 | CYP2D6-substrate: | 0.877 |
| CYP3A4-inhibitor: | 0.118 | CYP3A4-substrate: | 0.572 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 10.204 | Half-life (T1/2): | 0.82 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.046 | Human Hepatotoxicity (H-HT): | 0.51 |
| Drug-inuced Liver Injury (DILI): | 0.638 | AMES Toxicity: | 0.935 |
| Rat Oral Acute Toxicity: | 0.044 | Maximum Recommended Daily Dose: | 0.048 |
| Skin Sensitization: | 0.46 | Carcinogencity: | 0.863 |
| Eye Corrosion: | 0.057 | Eye Irritation: | 0.886 |
| Respiratory Toxicity: | 0.035 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000223 | 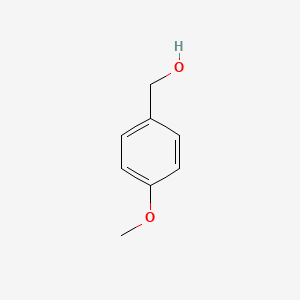 |
0.590 | D05CKR |  |
0.344 | ||
| ENC000310 | 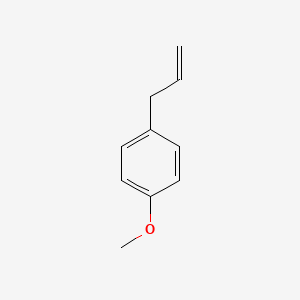 |
0.585 | D02HXS |  |
0.339 | ||
| ENC005495 | 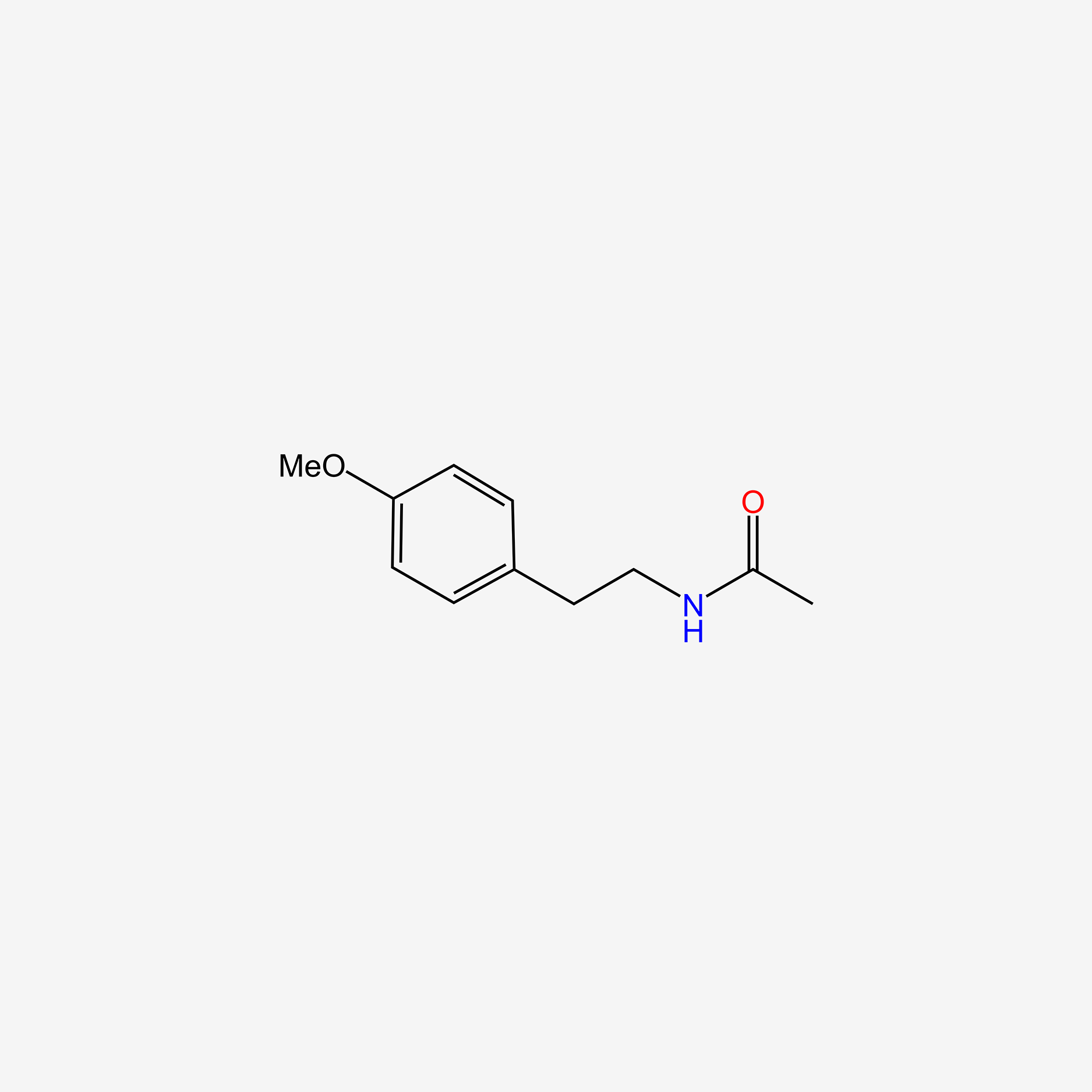 |
0.490 | D02DPU |  |
0.339 | ||
| ENC000318 | 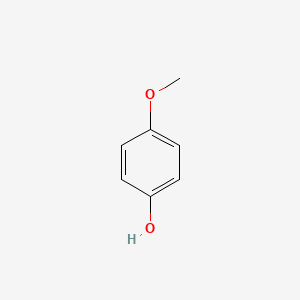 |
0.475 | D04KJO |  |
0.333 | ||
| ENC000221 |  |
0.475 | D0Q1IT |  |
0.333 | ||
| ENC000298 | 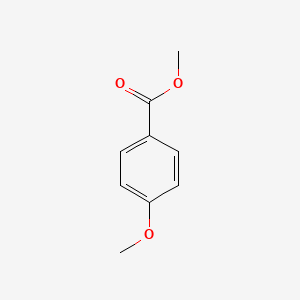 |
0.457 | D0D1DI |  |
0.333 | ||
| ENC000201 |  |
0.455 | D0I2MK | 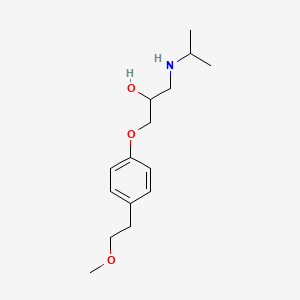 |
0.318 | ||
| ENC001460 | 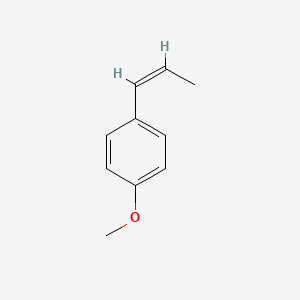 |
0.444 | D08JZS | 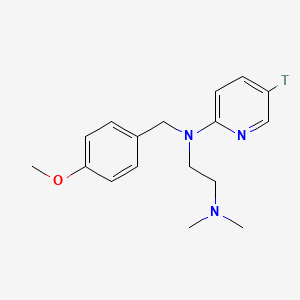 |
0.315 | ||
| ENC000740 |  |
0.442 | D03XTC |  |
0.314 | ||
| ENC001578 | 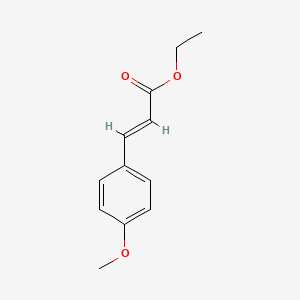 |
0.434 | D0J5DC | 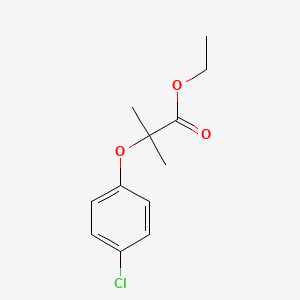 |
0.305 | ||