NPs Basic Information

|
Name |
4-Methylcatechol
|
| Molecular Formula | C7H8O2 | |
| IUPAC Name* |
4-methylbenzene-1,2-diol
|
|
| SMILES |
CC1=CC(=C(C=C1)O)O
|
|
| InChI |
InChI=1S/C7H8O2/c1-5-2-3-6(8)7(9)4-5/h2-4,8-9H,1H3
|
|
| InChIKey |
ZBCATMYQYDCTIZ-UHFFFAOYSA-N
|
|
| Synonyms |
4-Methylcatechol; 452-86-8; 4-methylbenzene-1,2-diol; 3,4-Dihydroxytoluene; Homocatechol; p-Methylcatechol; 4-METHYLPYROCATECHOL; 4-Methyl-1,2-benzenediol; Homopyrocatechol; Toluene-3,4-diol; 1,2-Dihydroxy-4-methylbenzene; p-Methylpyrocatechol; 1,2-Benzenediol, 4-methyl-; 4-Methyl-1,2-dihydroxybenzene; Pyrocatechol, 4-methyl-; 2-Hydroxy-4-methylphenol; CHEBI:17254; NSC 17489; 1-Methyl-3,4-dihydroxybenzene; 12GLI7JGB3; CHEMBL158766; MFCD00002205; NSC-17489; 5-methylcatechol; MCT; CCRIS 3333; EINECS 207-214-5; UNII-12GLI7JGB3; BRN 0636512; 4-Methylcatehol; 4-Metylcatechol; 4-methyl catechol; 4-methyl-catechol; 4-methyl pyrocatechol; 4-methyl-Pyrocatechol; 4k7n; DSSTox_CID_861; bmse000475; Epitope ID:150928; DSSTox_RID_75831; 4-methyl-benzene-1,2-diol; DSSTox_GSID_20861; SCHEMBL12388; 4-Methylcatechol, >=95%; METHYL CATECHOL, 4-; MLS001066329; 4-Methylbenzene-1,2-diol #; 1,2-dihydroxy-5-methylbenzene; DTXSID5020861; 4-methyl-1,2-dihydroxy benzene; HMDB00873; HMS3886M12; AMY25678; NSC17489; Tox21_200632; BDBM50548723; c0126; s5616; ZINC13512210; AKOS000121479; CCG-266087; CS-W013530; DB04120; HY-W012814; NCGC00248773-01; NCGC00258186-01; AC-12438; AS-11944; CAS-452-86-8; SMR000471857; SY012747; DB-051291; FT-0618974; M0413; EN300-21094; C06730; D70562; A872403; DROXIDOPA METABOLITE (3,4-DIHYDROXYTOLUENE); W-106144; Q15303189; F0001-1227; Z104490128
|
|
| CAS | 452-86-8 | |
| PubChem CID | 9958 | |
| ChEMBL ID | CHEMBL158766 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 124.14 | ALogp: | 1.4 |
| HBD: | 2 | HBA: | 2 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 40.5 | Aromatic Rings: | 1 |
| Heavy Atoms: | 9 | QED Weighted: | 0.518 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.44 | MDCK Permeability: | 0.00001790 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.06 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.869 |
| 30% Bioavailability (F30%): | 0.765 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.066 | Plasma Protein Binding (PPB): | 73.34% |
| Volume Distribution (VD): | 0.507 | Fu: | 18.21% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.744 | CYP1A2-substrate: | 0.899 |
| CYP2C19-inhibitor: | 0.18 | CYP2C19-substrate: | 0.155 |
| CYP2C9-inhibitor: | 0.107 | CYP2C9-substrate: | 0.809 |
| CYP2D6-inhibitor: | 0.315 | CYP2D6-substrate: | 0.848 |
| CYP3A4-inhibitor: | 0.06 | CYP3A4-substrate: | 0.233 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 19.151 | Half-life (T1/2): | 0.918 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.011 | Human Hepatotoxicity (H-HT): | 0.039 |
| Drug-inuced Liver Injury (DILI): | 0.083 | AMES Toxicity: | 0.524 |
| Rat Oral Acute Toxicity: | 0.825 | Maximum Recommended Daily Dose: | 0.292 |
| Skin Sensitization: | 0.935 | Carcinogencity: | 0.637 |
| Eye Corrosion: | 0.974 | Eye Irritation: | 0.989 |
| Respiratory Toxicity: | 0.854 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000734 |  |
0.545 | D06GIP |  |
0.514 | ||
| ENC000172 |  |
0.545 | D0T7OW |  |
0.500 | ||
| ENC000002 |  |
0.514 | D04PHC | 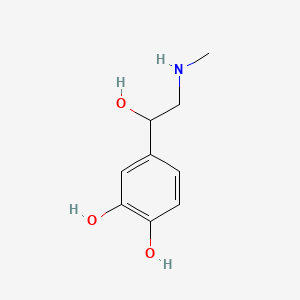 |
0.475 | ||
| ENC000404 |  |
0.500 | D07MOX | 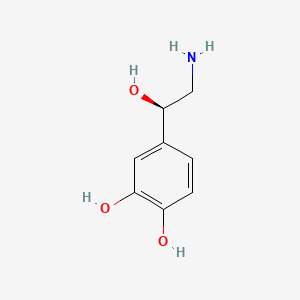 |
0.474 | ||
| ENC002095 |  |
0.475 | D0V9EN | 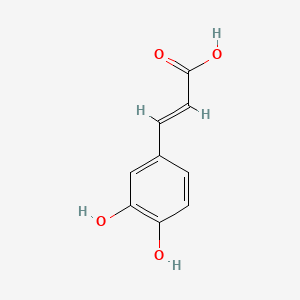 |
0.439 | ||
| ENC000035 |  |
0.474 | D0BA6T | 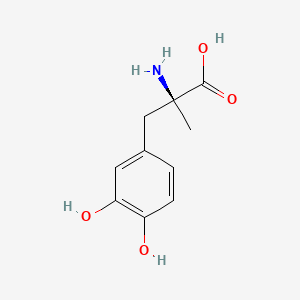 |
0.432 | ||
| ENC001440 |  |
0.439 | D0U0OT | 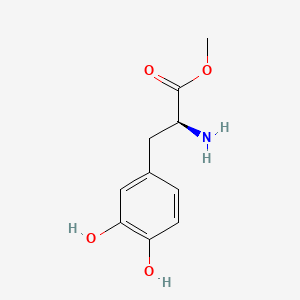 |
0.422 | ||
| ENC000021 |  |
0.438 | D0I8FI | 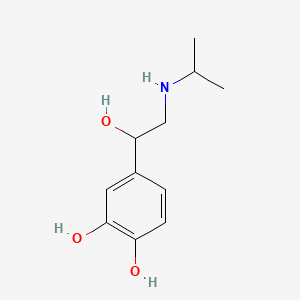 |
0.422 | ||
| ENC000344 | 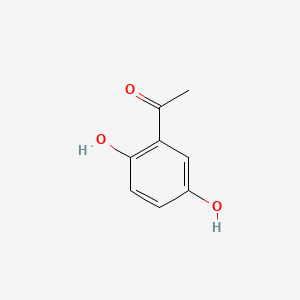 |
0.432 | D08HVR | 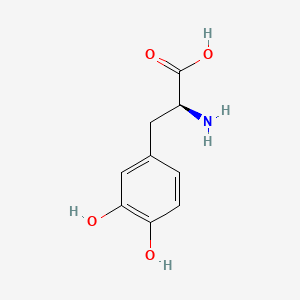 |
0.419 | ||
| ENC000127 |  |
0.419 | D0P7JZ | 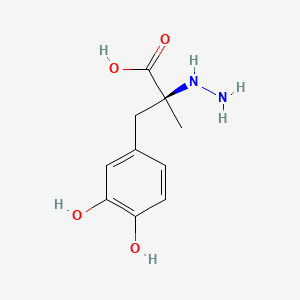 |
0.404 | ||