NPs Basic Information

|
Name |
3,4-Dihydroxybenzoic acid
|
| Molecular Formula | C7H6O4 | |
| IUPAC Name* |
3,4-dihydroxybenzoic acid
|
|
| SMILES |
C1=CC(=C(C=C1C(=O)O)O)O
|
|
| InChI |
InChI=1S/C7H6O4/c8-5-2-1-4(7(10)11)3-6(5)9/h1-3,8-9H,(H,10,11)
|
|
| InChIKey |
YQUVCSBJEUQKSH-UHFFFAOYSA-N
|
|
| Synonyms |
3,4-DIHYDROXYBENZOIC ACID; protocatechuic acid; 99-50-3; 4-Carboxy-1,2-dihydroxybenzene; Protocatehuic acid; protocatechuate; Benzoic acid, 3,4-dihydroxy-; 4,5-Dihydroxybenzoic acid; 3,4-Dihydroxybenzoicacid; protocatechuicacid; MFCD00002509; Catechol-4-carboxylic Acid; CCRIS 6291; 3, 4-Dihydroxybenzoic acid; CHEMBL37537; MLS000737807; 36R5QJ8L4B; EINECS 202-760-0; CHEBI:36062; NSC 16631; BRN 1448841; NSC-16631; 1ykp; 3,4-Dihydroxy Benzoic Acid; NSC16631; DB03946; SMR000528167; UNII-36R5QJ8L4B; C00230; D-3487; Hypogallic acid; b-Resorcylate; beta-Resorcylate; b-resorcylic acid; 4fht; Protacatechuic Acid; ZINCSELENITE; Carbohydroquinonic acid; cid_72; Protocatechuic Acid,(S); PROTOCATECHOIC ACID; DSSTox_CID_1212; Protocatechuic acid (M1); bmse000328; 3,4-dihydroxy-benzoic acid; DSSTox_RID_76012; DSSTox_GSID_21212; SCHEMBL39435; 3,4-Dihydroxybenzoate, VIII; Pyrocatechol-4-carboxylic Acid; DTXSID4021212; FEMA NO. 4430; PROTOCATECHUIC ACID [MI]; ZINC13246; PROTOCATECHUIC ACID (PCA); HMS2270A17; KUC104409N; ACT07872; HY-N0294; Tox21_200167; BBL012232; BDBM50100861; DIHYDROXYBENZOIC ACID, 3,4-; s3975; STL163570; AKOS000119632; AC-9617; CCG-207950; CS-6092; KSC-10-128; CAS-99-50-3; NCGC00246757-01; NCGC00246757-02; NCGC00257721-01; 3,4-DIHYDROXYBENZOIC ACID [INCI]; AS-10808; SY014104; DB-021903; AM20060767; FT-0600028; 3,4-Dihydroxybenzoic acid, >=97.0% (T); EN300-21544; 3,4-Dihydroxybenzoic acid, analytical standard; F11285; 3,4-dihydroxybenzoate;3,4-Dihydroxybenzoic acid; 002D509; A846038; AE-562/40524392; DROXIDOPA METABOLITE (PROTOCATECHOIC ACID); Q418599; 976C8CCE-B25D-4E0A-9A6F-3CEEA7A6964F; Z104501142; 3,4-Dihydroxybenzoic acid, Vetec(TM) reagent grade, 97%; Protocatechuic acid, primary pharmaceutical reference standard; PROTOCATECHOIC ACID (CONSTITUENT OF MARITIME PINE) [DSC]; Protocatechuic acid, United States Pharmacopeia (USP) Reference Standard; 1225528-47-1
|
|
| CAS | 99-50-3 | |
| PubChem CID | 72 | |
| ChEMBL ID | CHEMBL37537 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 154.12 | ALogp: | 1.1 |
| HBD: | 3 | HBA: | 4 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 77.8 | Aromatic Rings: | 1 |
| Heavy Atoms: | 11 | QED Weighted: | 0.531 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.469 | MDCK Permeability: | 0.00000639 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.002 |
| Human Intestinal Absorption (HIA): | 0.032 | 20% Bioavailability (F20%): | 0.367 |
| 30% Bioavailability (F30%): | 0.97 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.185 | Plasma Protein Binding (PPB): | 41.69% |
| Volume Distribution (VD): | 0.395 | Fu: | 48.11% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.033 | CYP1A2-substrate: | 0.086 |
| CYP2C19-inhibitor: | 0.034 | CYP2C19-substrate: | 0.043 |
| CYP2C9-inhibitor: | 0.117 | CYP2C9-substrate: | 0.102 |
| CYP2D6-inhibitor: | 0.014 | CYP2D6-substrate: | 0.124 |
| CYP3A4-inhibitor: | 0.045 | CYP3A4-substrate: | 0.036 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 11.905 | Half-life (T1/2): | 0.941 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.023 | Human Hepatotoxicity (H-HT): | 0.485 |
| Drug-inuced Liver Injury (DILI): | 0.842 | AMES Toxicity: | 0.068 |
| Rat Oral Acute Toxicity: | 0.112 | Maximum Recommended Daily Dose: | 0.006 |
| Skin Sensitization: | 0.452 | Carcinogencity: | 0.046 |
| Eye Corrosion: | 0.205 | Eye Irritation: | 0.973 |
| Respiratory Toxicity: | 0.783 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000296 |  |
0.605 | D0V9EN | 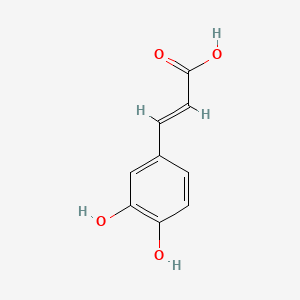 |
0.561 | ||
| ENC000035 |  |
0.605 | D08HVR | 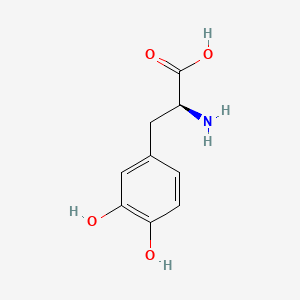 |
0.535 | ||
| ENC000097 | 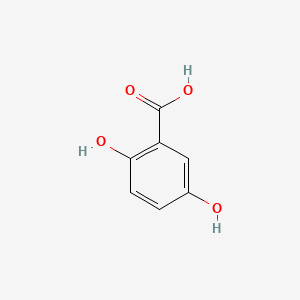 |
0.568 | D0BA6T | 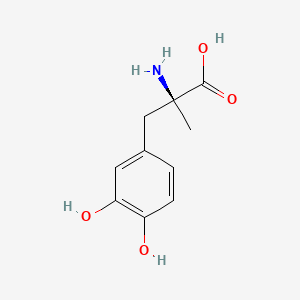 |
0.511 | ||
| ENC001440 |  |
0.561 | D0I3RO | 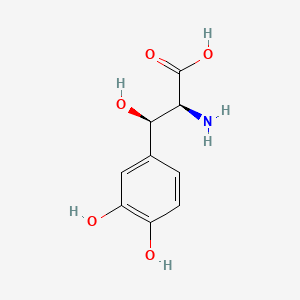 |
0.511 | ||
| ENC000029 |  |
0.538 | D0C4YC | 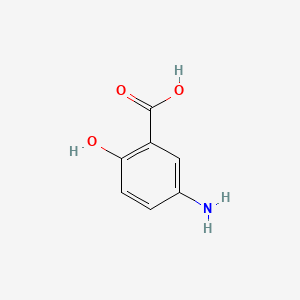 |
0.487 | ||
| ENC000127 |  |
0.535 | D0P7JZ | 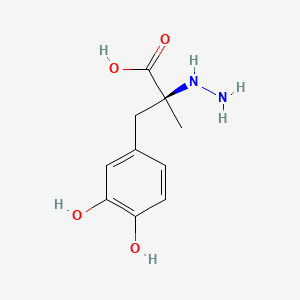 |
0.479 | ||
| ENC000069 |  |
0.526 | D07MOX | 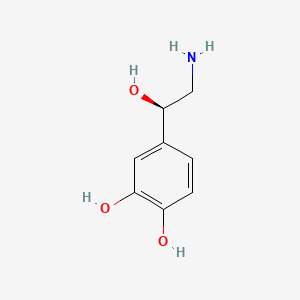 |
0.452 | ||
| ENC000329 |  |
0.514 | D01WJL | 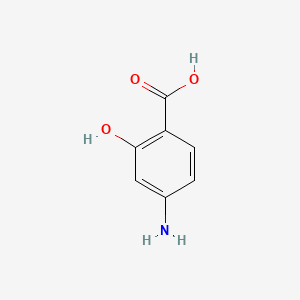 |
0.450 | ||
| ENC004146 |  |
0.511 | D0T7OW |  |
0.439 | ||
| ENC001090 |  |
0.500 | D0U0OT | 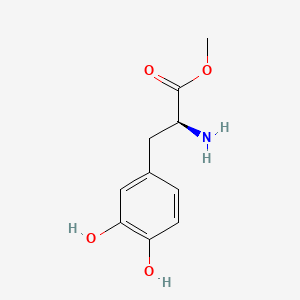 |
0.438 | ||