NPs Basic Information

|
Name |
3,4-Dihydroxyphenylacetic acid
|
| Molecular Formula | C8H8O4 | |
| IUPAC Name* |
2-(3,4-dihydroxyphenyl)acetic acid
|
|
| SMILES |
C1=CC(=C(C=C1CC(=O)O)O)O
|
|
| InChI |
InChI=1S/C8H8O4/c9-6-2-1-5(3-7(6)10)4-8(11)12/h1-3,9-10H,4H2,(H,11,12)
|
|
| InChIKey |
CFFZDZCDUFSOFZ-UHFFFAOYSA-N
|
|
| Synonyms |
3,4-Dihydroxyphenylacetic acid; 102-32-9; Dopac; 2-(3,4-DIHYDROXYPHENYL)ACETIC ACID; Homoprotocatechuic acid; Dopacetic acid; 3,4-Dihydroxybenzeneacetic acid; Benzeneacetic acid, 3,4-dihydroxy-; Dihydroxyphenylacetic acid; (3,4-DIHYDROXYPHENYL)ACETIC ACID; Homoprotocatechuate; Acetic acid, (3,4-dihydroxyphenyl)-; 3,4-Dihydroxy-phenylacetic acid; 3,4-dihydroxyphenylacetate; BA 2773; 3,4-DIHYDROXYPHENYLACETICACID; MFCD00004338; Lopac-D-9128; KEX5N0R4N5; 3,4-DHPOP; MLS001056737; 3,4-dihydroxyphenyl acetic acid; CHEBI:41941; NSC-73191; 3,4-DIHYDROXYPHENYLCAETIC ACID; SMR000326727; 4-Carboxymethylcatechol; DHY; 3,4-Dihydroxyphenylacetate, XV; 2-(3,4-dihydroxyphenyl)acetate; CCRIS 3765; Catechol-4-acetic Acid; EINECS 203-024-1; NSC 73191; UNII-KEX5N0R4N5; BRN 2211017; Dopacetate; AI3-52339; 3pcn; Dihydroxyphenylacetate; 1ai4; cid_547; 4-Carboxymethylpyrocatechol; bmse000329; Pyrocatechol-4-acetic Acid; 3,4-Dihydroxybenzeneacetate; CHEMBL1284; NCIOpen2_000518; Lopac0_000414; SCHEMBL36348; Benzeneacetic acid,4-dihydroxy-; DTXSID9074430; Acetic acid,4-dihydroxyphenyl)-; BDBM52946; HMDB01336; 3,4-dihydroxy-Benzeneacetic acid; 3,4-dihydroxyl phenylacetic acid; HMS2233I20; HMS3261C10; HMS3373A01; KUC106695N; KUC106697N; ZINC388555; (3,4-dihydroxyphenyl)-Acetic acid; NSC73191; Tox21_500414; s5639; 3,4-Dihydroxyphenylacetic acid, 98%; AKOS015890264; AC-5292; CCG-204506; CS-W001080; DB01702; Dopac/(3,4-dihydroxyphenyl)-aceticaci; HY-W001080; LP00414; RS-1018; SDCCGSBI-0050399.P002; Acetic acid, (3,4-dihydroxyphenyl)-,; NCGC00015381-01; NCGC00015381-02; NCGC00015381-03; NCGC00015381-04; NCGC00015381-05; NCGC00015381-06; NCGC00093838-01; NCGC00093838-02; NCGC00093838-03; NCGC00261099-01; HAA; KSC-11-207-5; KSC-11-207-8; SY015718; 2-[3,4-bis(oxidanyl)phenyl]ethanoic acid; AM20020093; D1283; EU-0100414; FT-0614331; 3,4-Dihydroxyphenylacetic Acid-[13C,18O2]; C01161; D 9128; EN300-111909; A800559; SR-01000075841; 3,4-Dihydroxyphenylacetic acid, analytical standard; J-000672; Q4634071; SR-01000075841-1; Z1255438427; 47115C80-8C82-419A-BC51-B10A2CB7FE8F
|
|
| CAS | 102-32-9 | |
| PubChem CID | 547 | |
| ChEMBL ID | CHEMBL1284 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 168.15 | ALogp: | 0.5 |
| HBD: | 3 | HBA: | 4 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 77.8 | Aromatic Rings: | 1 |
| Heavy Atoms: | 12 | QED Weighted: | 0.578 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.317 | MDCK Permeability: | 0.00001840 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.006 |
| Human Intestinal Absorption (HIA): | 0.041 | 20% Bioavailability (F20%): | 0.573 |
| 30% Bioavailability (F30%): | 0.045 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.071 | Plasma Protein Binding (PPB): | 58.46% |
| Volume Distribution (VD): | 0.337 | Fu: | 33.64% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.029 | CYP1A2-substrate: | 0.084 |
| CYP2C19-inhibitor: | 0.035 | CYP2C19-substrate: | 0.056 |
| CYP2C9-inhibitor: | 0.052 | CYP2C9-substrate: | 0.853 |
| CYP2D6-inhibitor: | 0.017 | CYP2D6-substrate: | 0.235 |
| CYP3A4-inhibitor: | 0.018 | CYP3A4-substrate: | 0.073 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 17.407 | Half-life (T1/2): | 0.94 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.012 | Human Hepatotoxicity (H-HT): | 0.2 |
| Drug-inuced Liver Injury (DILI): | 0.836 | AMES Toxicity: | 0.638 |
| Rat Oral Acute Toxicity: | 0.103 | Maximum Recommended Daily Dose: | 0.007 |
| Skin Sensitization: | 0.801 | Carcinogencity: | 0.17 |
| Eye Corrosion: | 0.903 | Eye Irritation: | 0.964 |
| Respiratory Toxicity: | 0.1 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000862 |  |
0.684 | D08HVR | 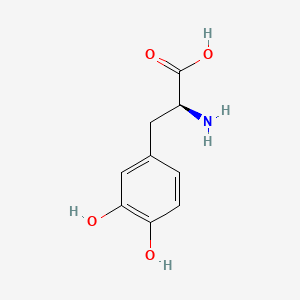 |
0.643 | ||
| ENC002095 |  |
0.675 | D0BA6T | 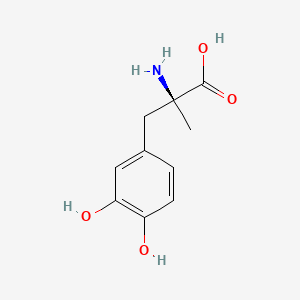 |
0.614 | ||
| ENC000127 |  |
0.643 | D0P7JZ | 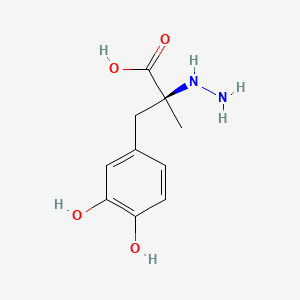 |
0.574 | ||
| ENC000002 |  |
0.605 | D0T7OW |  |
0.550 | ||
| ENC001440 |  |
0.523 | D0U0OT | 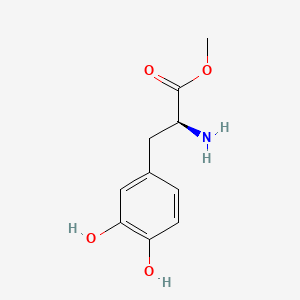 |
0.532 | ||
| ENC000006 |  |
0.512 | D0V9EN | 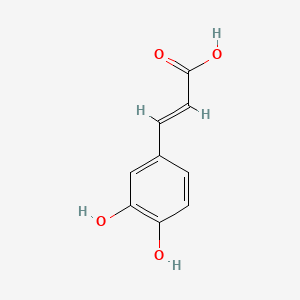 |
0.523 | ||
| ENC002317 |  |
0.488 | D0I3RO | 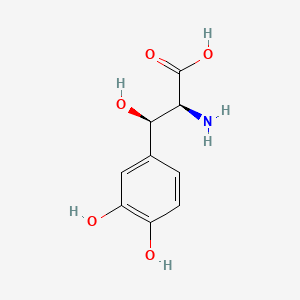 |
0.479 | ||
| ENC000097 | 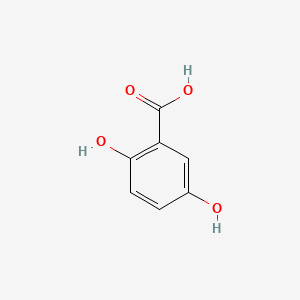 |
0.488 | D0Y6KO | 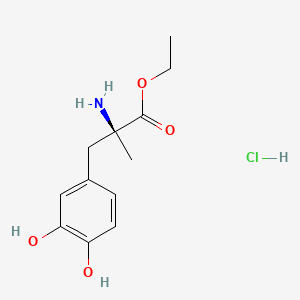 |
0.472 | ||
| ENC000329 |  |
0.474 | D07MOX | 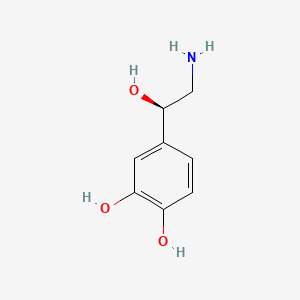 |
0.455 | ||
| ENC000069 |  |
0.452 | D0C6OQ |  |
0.431 | ||