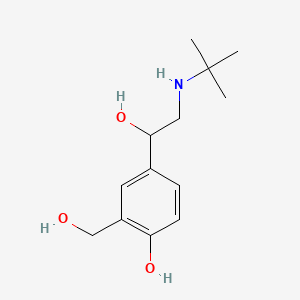
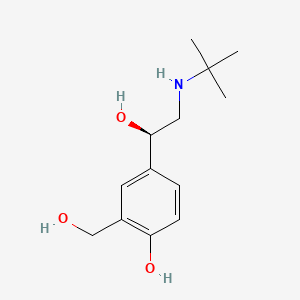
| Synonyms |
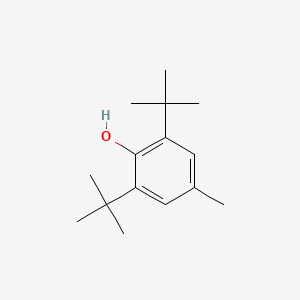
119-47-1; 2,2'-Methylenebis(4-methyl-6-tert-butylphenol); Antioxidant 2246; 2,2'-Methylenebis(6-tert-butyl-4-methylphenol); 2,2'-Methylenebis(6-tert-butyl-p-cresol); Bisalkofen BP; 6,6'-Methylenebis(2-(tert-butyl)-4-methylphenol); Antioxidant BKF; Antioxidant 1; Anti Ox; Chemanox 21; Catolin 14; Advastab 405; Nocrac NS 6; Plastanox 2246; Vulkanox bkf; Bisaklofen BP; Lederle 2246; Sumilizer MDP; AO 1 (Antioxidant); GERI-BP002-A; Synox 5lt; Antioxidant NG-2246; Alterungsschutzmittel BKF; Calco 2246; Methylenebis; CAO 5; Plastanox 2246 Antioxidant; Antage W 400; Cyanox 2246; CAO 14; methylene di-t-butylcresol; Phenol, 2,2'-methylenebis[6-(1,1-dimethylethyl)-4-methyl-; OXY CHEK 114; AO 2246; NG 2246; Antioxidant OMB; Nocrack NS 6; 6,6'-Di-tert-butyl-2,2'-methylenedi-p-cresol; 2,2'-Bis-6-terc.butyl-p-kresylmethan; 2,2'-Methylene-bis(6-tert-butyl-4-methylphenol); A-22-46; Di(2-hydroxy-5-methyl-3-tert-butylphenyl)methane; Lowinox 22M46; Methane, 2,2'-bis(6-t-butyl-p-cresyl)-; p-Cresol, 2,2'-methylenebis(6-tert-butyl-; 2-tert-butyl-6-[(3-tert-butyl-2-hydroxy-5-methylphenyl)methyl]-4-methylphenol; BIS(2-HYDROXY-3-TERT-BUTYL-5-METHYLPHENYL)METHANE; Methane, 2,2'-bis(6-tert-butyl-p-cresyl)-; Bis(6-hydroxy-3-methyl-5-tert-butylphenyl)methane; KVM0X4X57B; 2,2'-Methylenebis(6-(1,1-dimethylethyl)-p-cresol); 2,2-methylenebis(6-tert-butyl-p-cresol); 2,2'-Methylenebis[6-tert-butyl-p-cresol]; AO 1; CHEMBL460648; BKF; DTXSID4020870; Phenol, 2,2'-methylenebis(6-(1,1-dimethylethyl)-4-methyl-; 2,2-methylenebis(6-tert-butyl-4-methylphenol); 2,2'-Methylenebis(6-tert-butyl-4-methyl-phenol); NSC-7781; DSSTox_CID_870; p-Cresol, 2,2'-methylenebis[6-tert-butyl-; 2,2'-Bis(4-methyl-6-tert-butylphenol)methane; 2,2'-Methylenebis(4-methyl-6-t-butylphenol); 2,2'-methylene-bis(4-methyl-6-t-butylphenol); DSSTox_RID_75838; 2,2'-Methylenebis[4-Methyl-6-tert-butylphenol]; DSSTox_GSID_20870; 2,2'-methanediylbis[6-(1,1-dimethylethyl)-4-methylphenol]; 3,3'-Di-tert-butyl-2,2'-dihydroxy-5,5'-dimethyldiphenylmethane; 2-tert-Butyl-6-(2-hydroxy-3-tert-butyl-5-methyl-benzyl)-4-methyl-phenol; CAS-119-47-1; AO 1 (VAN); CCRIS 4919; HSDB 5585; Methylene bis methyl butyl phenol; NSC 7781; EINECS 204-327-1; UNII-KVM0X4X57B; BRN 2062676; AI3-18027; 2,2-Methylenebis(4-methyl-6-t-butylphenol); 2,2'-Bis-6-terc.butyl-p-kresylmethan [Czech]; Agidol 2; A 22-46; Ionol 46; Ralox 46; Rubber Antioxidant 2246; p-Cresol, 2,2'-methylenebis(6-tert- butyl-; EC 204-327-1; CAO-5; Oprea1_122036; SCHEMBL34162; 2,2'-methylene-bis-(4-methyl-6-tert-butylphenol); 4-06-00-06801 (Beilstein Handbook Reference); BIDD:ER0324; 2,1-dimethylethyl)-p-cresol]; CAO-14; NSC7781; CHEBI:172336; ZINC1543799; Tox21_201529; Tox21_302923; BDBM50522651; MFCD00043641; STL377901; AKOS000447157; CCG-207916; CCG-208597; s12392; 2,2'-methylenebis(6-t-butyl-p-cresol); NCGC00164172-01; NCGC00164172-02; NCGC00256347-01; NCGC00259079-01; p-Cresol,2'-methylenebis[6-tert-butyl-; AS-13205; Methane,2'-bis(6-tert-butyl-p-cresyl)-; METHYLENE DI-T-BUTYLCRESOL [INCI]; 2,2'Methylenebis(6-tert-4-methylphenol); CS-0081317; FT-0609309; M0217; p-Cresol, 2,2'-methylenebis*6-tert-butyl-; 2,2'-methyl enebis(4-methyl-6-t-butylphenol); 2,2'-Methylene-bis(6-tert-butyl)-para-cresol; 2,2'-Methylenebis(6-t-butyl-4-methylphenol); MLS-0146298.0001; 2,2'-Methylene-bis(4-methyl-6-tert-butylphenol; 2,2'-Methylene-bis-(4-methyl-6-t-butylphenol); 6,6'-methylenebis(2-tert-butyl-4-methylphenol); 2,2'-methanediylbis(6-tert-butyl-4-methylphenol); 2,2'-methylene-bis(4-methyl-6-tert-butylphenol); 2,2'-methylene-bis(4-methyl-6-tert.butylphenol); 2,2'-methylenebis (6-tert-butyl-4-methylphenol); A804292; bis(2-hydroxy-5-methyl-3-tert-butylphenyl)methane; Bis(3-tert-butyl-2-hydroxy-5-methylphenyl)methane; 2,2'- methylene bis(4-methyl-6-tert. butylphenol); 2,2'-methylene-bis(4-methyl-6-tertiarybutylphenol); 2,2'-methylene-bis-(4-methyl-6-tert.butylphenol); 2,2'-methylene-bis-(6-tert-butyl-4-methylphenol); 2,2'-METHYLENEBIS-(6-TERT-BUTYL-P-CRESOL); J-004134; METHYLENEBIS(6-TERT-BUTYL-P-CRESOL), 2,2'-; Q16830225; 2,2'-Methylenebis 6-(1,1-dimethylethyl)-4-methyl-phenol; 2,2'-METHYLENEBIS(6-TERT.BUTYL-4-METHYL-PHENOL); Phenol,2'-methylenebis[6-(1,1-dimethylethyl)-4-methyl-; WLN: 1X1 & 1 & R BQ C3R BQ CX1 & 1 & 1; P-CRESOL, 2,2'-METHYLENEBIS(6-TERT- BUTYL- [HSDB]; Phenol, 2,2'-methylenebis*6-(1,1-dimethylethyl)-4-methyl-; 2,2'-METHYLENEBIS(6-(1,1-DIMETHYLETHYL)-4-METHYL)PHENOL; 6,6'-DI-TERT-BUTYL-4,4'-DIMETHYL-2,2'-METHYLENEDIPHENOL
|