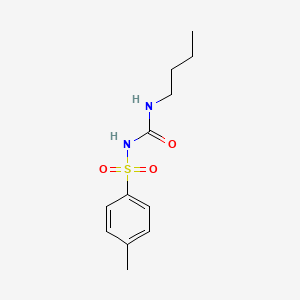
tolbutamide, 64-77-7, Orinase, Arkozal, Willbutamide, Butamide, Diabetamid, Ipoglicone, Glyconon, Rastinon, Tolbutamid, Aglicid, Artosin, Diaben, Tolumid, 1-Butyl-3-tosylurea, Diasulfon, Artozin, Butamid, Diabetol, Diabuton, Dirastan, Dolipol, Mobenol, Oterben, Pramidex, Tolbusal, Toluina, Toluvan, Tolylsulfonylbutylurea, 1-Butyl-3-(p-tolylsulfonyl)urea, Drabet, Orabet, Oralin, Orezan, Orinaz, Tolbutamidum, Sk-tolbutamide, N-(Butylcarbamoyl)-4-methylbenzenesulfonamide, N-n-Butyl-N'-tosylurea, Tolbutamida, 1-p-Toluenesulfonyl-3-butylurea, 1-Butyl-3-(p-methylphenylsulfonyl)urea, Arcosal, Beglucin, Butamidum, Diabesan, N-Butyl-N'-(p-tolylsulfonyl)urea, Tarasina, Tolbutone, Tolbet, N-Butyl-N'-p-toluenesulfonylurea, 3-(p-Tolyl-4-sulfonyl)-1-butylurea, N-(4-Methylphenylsulfonyl)-N'-butylurea, N-Butyl-N'-(4-methylphenylsulfonyl)urea, N-(4-Methylbenzenesulfonyl)-N'-butylurea, HLS 831, N-(Sulfonyl-p-methylbenzene)-N'-N-butylurea, Orinase (TN), Tolumide, N-(p-Tolylsulfonyl)-N'-butylcarbamide, NCI-C01763, N-4-Methylbenzolsulfonyl-N-butylurea, Urea, 1-butyl-3-(p-tolylsulfonyl)-, D 860, Tolbutamidum [INN-Latin], 1-butyl-3-(4-methylphenyl)sulfonylurea, 1-Butyl-3-(4-methylphenylsulfonyl)urea, Benzenesulfonamide, N-[(butylamino)carbonyl]-4-methyl-, Tolbutamida [INN-Spanish], N-Butyl-N'-toluene-p-sulfonylurea, CCRIS 592, N-[(butylamino)carbonyl]-4-methylbenzenesulfonamide, N-4-(Methylbenzolsulfonyl)-n-butylurea, HSDB 3393, N-(p-Methylbenzenesulfonyl)-N'-butylurea, UNII-982XCM1FOI, EINECS 200-594-3, 982XCM1FOI, MFCD00027169, NSC 23813, NSC-23813, U 2043, BRN 1984428, 3-butyl-1-(4-methylbenzenesulfonyl)urea, DTXSID8021359, CHEBI:27999, N-(Sulfonyl-p-methylbenzene)-N'-butylurea, 3-butyl-1-[(4-methylbenzene)sulfonyl]urea, Benzenesulfonamide, N-((butylamino)carbonyl)-4-methyl-, NSC-87833, 1-BUTYL-3-(4-METHYLBENZENESULFONYL)UREA, CHEMBL782, N-((Butylamino)carbonyl)-4-methylbenzenesulfonamide, MLS000028399, DTXCID801359, Tolbutamide [USP:INN:BAN:JAN], 4-11-00-00396 (Beilstein Handbook Reference), CAS-64-77-7, toluran, NCGC00015999-10, SMR000058363, Tolbutamide form I^L^, Tolbutamidum (INN-Latin), Tolbutamida (INN-Spanish), TOLBUTAMIDE (MART.), TOLBUTAMIDE [MART.], TOLBUTAMIDE (USP-RS), TOLBUTAMIDE [USP-RS], TOLBUTAMIDE (EP MONOGRAPH), Tolbutamide (USP:INN:BAN:JAN), TOLBUTAMIDE [EP MONOGRAPH], TOLBUTAMIDE (USP MONOGRAPH), TOLBUTAMIDE [USP MONOGRAPH], SR-01000003059, tolbutamide IL, tolbutamide III, Prestwick_471, Spectrum_000447, Tolbutamide form I^H^, Opera_ID_112, TOLBUTAMIDE [MI], Prestwick0_000190, Prestwick1_000190, Prestwick2_000190, Prestwick3_000190, Spectrum2_001210, Spectrum3_000599, Spectrum4_000358, Spectrum5_001272, Lopac-T-0891, TOLBUTAMIDE [INN], TOLBUTAMIDE [JAN], N-n-Butyl-N''-tosylurea, TOLBUTAMIDE [HSDB], WLN: 4MVMSWR D1, T 0891, TOLBUTAMIDE [VANDF], NCIOpen2_009592, BIDD:PXR0179, CBiol_001920, Lopac0_001154, SCHEMBL15918, BSPBio_000119, BSPBio_001507, BSPBio_002078, KBioGR_000227, KBioGR_000795, KBioGR_002275, KBioSS_000227, KBioSS_000927, KBioSS_002276, TOLBUTAMIDE [WHO-DD], TOLBUTAMIDE [WHO-IP], MLS001148399, MLS002152944, DivK1c_000341, SPECTRUM1500581, SPBio_001000, SPBio_002040, BPBio1_000131, GTPL6848, Tolbutamide (JP17/USP/INN), Tolbutamide, analytical standard, HMS501B03, KBio1_000341, KBio2_000227, KBio2_000927, KBio2_002275, KBio2_002795, KBio2_003495, KBio2_004843, KBio2_005363, KBio2_006063, KBio2_007411, KBio3_000453, KBio3_000454, KBio3_001578, KBio3_002755, A10BB03, TOLBUTAMIDE [ORANGE BOOK], V04CA01, cMAP_000008, NINDS_000341, Bio1_000206, Bio1_000695, Bio1_001184, Bio2_000227, Bio2_000707, HMS1361L09, HMS1568F21, HMS1791L09, HMS1989L09, HMS2089C17, HMS2092M21, HMS2095F21, HMS2232H16, HMS3259A08, HMS3263H09, HMS3402L09, HMS3651N03, HMS3712F21, Pharmakon1600-01500581, BCP09192, HY-B0401, NSC23813, TOLBUTAMIDUM [WHO-IP LATIN], N-Butyl-N''-(p-tolylsulfonyl)urea, Tox21_110279, Tox21_201612, Tox21_302795, Tox21_501154, BDBM50027886, CCG-39141, NSC757354, NSC813220, s2443, 1-Butyl-3-(para-tolylsulfonyl)-urea, AKOS015894999, Tox21_110279_1, 1ST7197, DB01124, LP01154, NC00543, NSC-757354, NSC-813220, SDCCGSBI-0051121.P004, Tolbutamide 1.0 mg/ml in Acetonitrile, IDI1_000341, IDI1_033977, N-(n-Butyl)-N'-p-toluene-sulfonylurea, NCGC00015999-01, NCGC00015999-02, NCGC00015999-03, NCGC00015999-04, NCGC00015999-05, NCGC00015999-06, NCGC00015999-07, NCGC00015999-08, NCGC00015999-09, NCGC00015999-11, NCGC00015999-12, NCGC00015999-13, NCGC00015999-14, NCGC00015999-15, NCGC00015999-16, NCGC00015999-17, NCGC00015999-19, NCGC00015999-20, NCGC00015999-29, NCGC00022721-03, NCGC00022721-04, NCGC00022721-05, NCGC00022721-06, NCGC00022721-07, NCGC00022721-08, NCGC00022721-09, NCGC00022721-10, NCGC00256548-01, NCGC00259161-01, NCGC00261839-01, AC-12490, AS-14136, SY014891, N-(4-Methylphenylsulfonyl)-N''-butylurea, N-Butyl-N''-(4-methylphenylsulfonyl)urea, SBI-0051121.P003, DB-054728, AB00052110, EU-0101154, FT-0603265, N-(P-TOLYLSULFONYL)N'-BUTYLCARBAMIDE, NS00001371, SW196681-3, T3690, BIM-0051121.0001, C07148, D00380, D87667, EN300-124678, U-2043, AB00052110-16, AB00052110_17, AB00052110_18, N'-4-METHYLBENZENESULFONYL-N''-BUTYLUREA, Tolbutamide, VETRANAL(TM), analytical standard, A834879, Q414275, SR-01000003059-2, SR-01000003059-4, SR-01000003059-7, W-104820, BRD-K85119730-001-06-5, BRD-K85119730-001-17-2, N'-butyl-N-(p-tolylsulfonyl)carbamimidate;Tolbutamide, Z44591715, Tolbutamide, European Pharmacopoeia (EP) Reference Standard, 1-(([(Butylamino)carbonyl]amino)sulfonyl)-4-methylbenzene #, Tolbutamide, United States Pharmacopeia (USP) Reference Standard