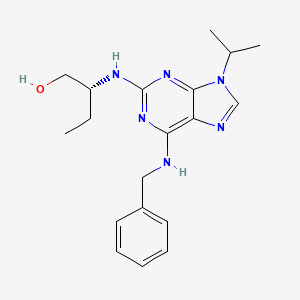
roscovitine, Seliciclib, 186692-46-6, R-Roscovitine, (R)-roscovitine, CYC202, CYC-202, CYC 202, Seliciclib [INN], (R)-2-((6-(Benzylamino)-9-isopropyl-9H-purin-2-yl)amino)butan-1-ol, 2-(R)-(1-Ethyl-2-hydroxyethylamino)-6-benzylamino-9-isopropylpurine, Seliciclib (Roscovitine), NSC-701554, AL-39256, ROSCOVITINE(Seliciclib), Roscovitin, CHEMBL14762, 0ES1C2KQ94, CHEBI:45307, (2R)-2-[[6-(benzylamino)-9-propan-2-ylpurin-2-yl]amino]butan-1-ol, (2R)-2-{[6-(benzylamino)-9-(propan-2-yl)-9H-purin-2-yl]amino}butan-1-ol, MFCD02266401, NSC701554, (2R)-2-[[6-(benzylamino)-9-isopropyl-purin-2-yl]amino]butan-1-ol, (2R)-2-((6-benzylamino-9-(propan-2-yl)-9h-purin-2-yl)amino)butan-1-ol, RRC, Rosco, Roscovitine (Seliciclib,CYC202), (2R)-2-{[6-(benzylamino)-9-(1-methylethyl)-9H-purin-2-yl]amino}butan-1-ol, NSC 701554, UNII-0ES1C2KQ94, 2-(1-ethyl-2-hydroxyethylamino)-6-benzylamino-9-isopropylpurine, BMK1-E12, 1unl, 3ddq, 1-Butanol, 2-((9-(1-methylethyl)-6-((phenylmethyl)amino)-9H-purin-2-yl)amino)-, (2R)-, 1-Butanol, 2-[[9-(1-methylethyl)-6-[(phenylmethyl)amino]-9H-purin-2-yl]amino]-, (2R)-, 2a4l, SELICICLIB [MI], BiomolKI_000048, 1-Butanol, (2R)-, BiomolKI2_000054, M02443, SELICICLIB [WHO-DD], CBiol_002016, Lopac0_001102, SCHEMBL94728, BSPBio_001078, KBioGR_000418, KBioSS_000418, MLS006011028, BDBM7533, cid_160355, GTPL6035, Roscovitine, >=98% (TLC), BCBcMAP01_000013, KBio2_000418, KBio2_002986, KBio2_005554, KBio3_000795, KBio3_000796, DTXSID20171928, EX-A052, BCPP000087, Bio1_000302, Bio1_000791, Bio1_001280, Bio2_000379, Bio2_000859, CC205, GLXC-04786, HMS1362F19, HMS1792F19, HMS1990F19, HMS3229N13, HMS3403F19, AMY10845, BCP01760, Roscovitine (Seliciclib, CYC202), HSCI1_000092, NSC800881, s1153, 6-(Benzylamino)-2(R)-[[1-(hydroxymethyl)propyl]amino]-9-isopropylpurine, AKOS005146319, AC-2416, CCG-100652, DB06195, NSC-800881, 2-(R)-[[9-(1-Methylethyl)-6-[(phenylmethyl)amino]-9H-purin-2-yl]amino]-1-butanol, IDI1_002134, Roscovitine - CAS 186692-46-6, SMP1_000266, 2,6,9-Trisubstituted purine deriv. 28, NCGC00094374-01, NCGC00094374-02, NCGC00094374-03, NCGC00094374-04, NCGC00094374-05, NCGC00094374-13, NCGC00094374-15, AS-56277, HY-30237, NCI60_036420, SMR004702823, FT-0674460, FT-0711432, NS00068708, SW220195-1, K00020, A813074, EN300-26484982, J-011999, J-524224, Q3494619, BRD-K07691486-001-03-1, BRD-K07691486-001-05-6, Roscovitine , R-roscovitine , CYC202, Z1741982636, (2R)-2-[[6-(benzylamino)-9-propan-2-yl-purin-2-yl]amino]butan-1-ol, (2R)-2-((9-(1-methylethyl)-6-((phenylmethyl)amino)-9H-purin-2-yl)amino)-1-butanol, (2R)-2-[[6-[(phenylmethyl)amino]-9-propan-2-yl-2-purinyl]amino]-1-butanol, (2R)-2-[[6-[(phenylmethyl)amino]-9-propan-2-yl-purin-2-yl]amino]butan-1-ol, (R)-2-((9-(1-methylethyl)-6-((phenylmethyl)amino)-9H-purin-2-yl)amino)-1-butanol, 1-Butanol, 2-((9-(1-methylethyl)-6-((phenylmethyl)amino)-9H-purin-2-yl)amino)-, (R)-, 2-[[9-(1-Methylethyl)-6-[(phenylmethyl)amino]- 9H-purin-2-yl]amino]-(R)-1-butanol