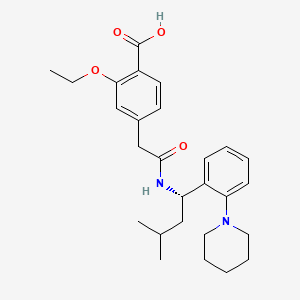
Repaglinide, 135062-02-1, Prandin, NovoNorm, GlucoNorm, AG-EE 623 ZW, Repaglinidum, Repaglinida, AG-EE 388 ZW, Repaglinidum [INN-Latin], Repaglinida [INN-Spanish], AGEE-623ZW, (S)-2-Ethoxy-4-(2-((3-methyl-1-(2-(piperidin-1-yl)phenyl)butyl)amino)-2-oxoethyl)benzoic acid, Surepost, Reglin, C27H36N2O4, AG-EE-623ZW, NSC-759893, UNII-668Z8C33LU, AG-EE-623-ZW, DTXSID3023552, A10BX02, 668Z8C33LU, (S)-(+)-2-Ethoxy-4-[N-[1-(2-piperidinophenyl)-3-methyl-1-butyl]aminocarbonylmethyl]benzoic acid, 2-ethoxy-4-[2-[[(1S)-3-methyl-1-(2-piperidin-1-ylphenyl)butyl]amino]-2-oxoethyl]benzoic acid, CHEMBL1272, (+)-2-Ethoxy-alpha-(((S)-alpha-isobutyl-o-piperidinobenzyl)carbamoyl)-p-toluic acid, (S)-2-ethoxy-4-(2-(3-methyl-1-(2-(piperidin-1-yl)phenyl)butylamino)-2-oxoethyl)benzoic acid, CHEBI:8805, DTXCID603552, (-)-Repaglinide, Repaglinide [USAN:USP:INN:BAN], MFCD00906179, 2-ethoxy-4-({[(1S)-3-methyl-1-[2-(piperidin-1-yl)phenyl]butyl]carbamoyl}methyl)benzoic acid, NSC 759893, NCGC00016978-01, Repaglinide [USAN], repa-glinide, CAS-135062-02-1, Repaglinidum (INN-Latin), Repaglinida (INN-Spanish), REPAGLINIDE (MART.), REPAGLINIDE [MART.], REPAGLINIDE (USP-RS), REPAGLINIDE [USP-RS], 2-ethoxy-4-[2-({(1S)-3-methyl-1-[2-(piperidin-1-yl)phenyl]butyl}amino)-2-oxoethyl]benzoic acid, Benzoic acid, 2-ethoxy-4-(2-((3-methyl-1-(2-(1-piperidinyl)phenyl)butyl)amino)-2-oxoethyl)-, (S)-, Actulin, REPAGLINIDE (EP MONOGRAPH), REPAGLINIDE [EP MONOGRAPH], REPAGLINIDE (USP MONOGRAPH), REPAGLINIDE [USP MONOGRAPH], Repaglinide (USAN:USP:INN:BAN), (+)-repaglinide, (S)-2-ETHOXY-4-(2-(METHYL-1-(2-(1-PIPERIDINYL)PHENYL)BUTYLAMINO)-2-OXOETHYL)-BENZOIC ACID, (S)-2-Ethoxy-4-[2-[[3-methyl-1-[2-(piperidin-1-yl)phenyl]butyl]amino]-2-oxoethyl]benzoic acid, 2-ethoxy-4-(2-{[(1S)-3-methyl-1-(2-piperidin-1-ylphenyl)butyl]amino}-2-oxoethyl)benzoic acid, SMR000466305, Prandin (TN), AG-EE 623ZW, Repaglinide (JAN/USP/INN), AG-EE-623 ZW, AG-EE-388, Repaglinide,(S), SMP-508, Surepost (TN), BJX, Repaglinide- Bio-X, NN-623, (S)-2-ethoxy-4-(2-((3-methyl-1-(2-(1-piperidinyl)-phenyl)butyl)amino)-2-oxoethyl)-benzoic acid, 111GE012, REPAGLINIDE [MI], Prestwick0_001046, Prestwick1_001046, Prestwick2_001046, Prestwick3_001046, REPAGLINIDE [INN], REPAGLINIDE [JAN], REPAGLINIDE [VANDF], (S)-2-Ethoxy-4-(2-((methyl-1-(2-(1-piperidinyl)phenyl)butyl)amino)-2-oxoethyl)-benzoic acid, SCHEMBL16137, BSPBio_000972, REPAGLINIDE [WHO-DD], MLS000759407, MLS001076684, MLS001424111, MLS006011560, BIDD:GT0338, SPBio_002906, REPAGLINIDE [EMA EPAR], BPBio1_001070, GTPL6841, REPAGLINIDE [ORANGE BOOK], Repaglinide for system suitability, HMS1571A14, HMS2051N08, HMS2094C07, HMS2098A14, HMS2231M21, HMS3414D09, HMS3678D09, HMS3715A14, Pharmakon1600-01506035, BCP04250, Tox21_110721, AC-726, BDBM50153520, HB1106, NSC759893, s1426, STK629501, AKOS005561792, Repaglinide, >=98% (HPLC), solid, BS-1010, CCG-101013, CS-0979, DB00912, NC00263, NCGC00016978-02, NCGC00016978-04, NCGC00016978-05, NCGC00016978-15, BR164332, HY-15209, SBI-0206942.P001, AB00514019, AM20090697, FT-0631150, FT-0674344, FT-0674345, R0179, SW197344-4, C07670, D00594, F15001, AB00514019-09, AB00514019_10, AB00514019_11, A806877, SR-01000759404, Q-201663, Q2195995, SR-01000759404-4, BRD-K82846253-001-03-0, Z1501485370, Repaglinide, European Pharmacopoeia (EP) Reference Standard, Repaglinide, United States Pharmacopeia (USP) Reference Standard, (s)-(+)-2-ethoxy-4-(2-oxo-2-[(alpha-isobutyl-2-piperidinobenzyl)amino]ethyl)-benzoic acid, (S)-2-Ethoxy-4-(2-(3-methyl-1-(2-piperidinophenyl)butylamino)-2-oxoethyl)benzoic acid, Repaglinide for system suitability, European Pharmacopoeia (EP) Reference Standard, (+)-2-ETHOXY-.ALPHA.-(((S)-.ALPHA.-ISOBUTYL-O-PIPERIDINOBENZYL)CARBAMOYL)-P-TOLUIC ACID, (2S)-2-[[(2S)-2-[[(2S,3S)-2-[[(2S)-4-amino-2-[[(2S,3S)-2-[[(2S)-2-[[2-[[(2S)-2-[[(2S)-1-[(2S)-2-[[(2S)-2-amino-5-guanidino-pentanoyl]amino]-4-carboxy-butanoyl]pyrrolidine-2-carbonyl]amino]propanoyl]amino]acetyl]amino]-4-methyl-pentanoyl]amino]-3-methyl-pe;(S)-2-Ethoxy-4-[2-[[3-methyl-1-[2-(1-piperidyl)phenyl]butyl]amino]-2-oxoethyl]benzoic Acid, (S)-2-Ethoxy-4-(2-((3-methyl-1-(2-(piperidin-1-yl)phenyl)butyl)amino)-2-oxoethyl)benzoicacid, (S)-2-Ethoxy-4-[2-[[[(1S)-3-methyl-1-[2-(1-piperidinyl)phenyl]butyl]amino]-2-oxoethyl]benzoic Acid, (S)-2-Ethoxy-4-[N-(1-(2-piperidino-phenyl)-3-methyl-1-butyl)-aminocarbonylmethyl]-benzoic acid, 2-Ethoxy-4-[2-[[(1S)-3-methyl-1-[2-(1-piperidinyl)phenyl]butyl]amino]-2-oxoethyl]benz oic acid, 2-Ethoxy-4-{[(S)-3-methyl-1-(2-piperidin-1-yl-phenyl)-butylcarbamoyl]-methyl}-benzoic acid, Benzoic Acid, 2-ethoxy-4-(2-((3-methyl-1-(2-(1-piperidinyl)phenyl)butyl)amino)-2-oxoethyl)-,(S)-, Benzoic acid, 2-ethoxy-4-[2-[[(1S)-3-methyl-1-[2-(1-piperidinyl)phenyl]butyl]amino]-2-oxoethyl]-