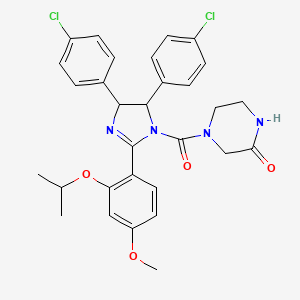
Nutlin-3, 548472-68-0, 890090-75-2, (Rac)-Nutlin-3, nutlin 3, 4-(4,5-bis(4-chlorophenyl)-2-(2-isopropoxy-4-methoxyphenyl)-4,5-dihydro-1H-imidazole-1-carbonyl)piperazin-2-one, Nutlin, 4-[[4,5-Bis(4-chlorophenyl)-4,5-dihydro-2-[4-methoxy-2-(1-methylethoxy)phenyl]-1H-imidazol-1-yl]carbonyl]-2-piperazinone, 4-[4,5-bis(4-chlorophenyl)-2-(4-methoxy-2-propan-2-yloxyphenyl)-4,5-dihydroimidazole-1-carbonyl]piperazin-2-one, (+/-)-Nutlin3, CHEMBL211045, MFCD07784509, C30H30Cl2N4O4, (+)-Nutlin-3, MFCD14636430, Nutlin 3(Random Configuration), NSC-732664, Rac-Nutlin-3, Nutln 3A, 4-({4,5-bis(4-chlorophenyl)-2-[4-methoxy-2-(propan-2-yloxy)phenyl]-4,5-dihydro-1H-imidazol-1-yl}carbonyl)piperazin-2-one, (?)-Nutlin-3, the p53/MDM2 Agonist, SCHEMBL2458627, BDBM31197, CHEBI:93777, EX-A851, BDUHCSBCVGXTJM-UHFFFAOYSA-N, HMS3651G03, HMS3653F08, HMS3653J08, HMS3750A11, AMY39899, BCP02265, BCP05161, BCP29278, HY-10029A, MFCD11977784, NSC732664, s1061, AKOS005146527, CCG-264800, SB19406, SB19407, NCGC00165848-01, NCGC00165848-02, (+/-)-4-[4,5-Bis(4-chlorophenyl)-2-(2-isopropoxy-4-methoxy-phenyl)-4,5-dihydro-imidazole-1-carbonyl]-piperazin-2-one, 4-{[4,5-Bis(4-chlorophenyl)-2-(2-isopropoxy-4-methoxyphenyl)-4,5- dihydro-1H-imidazol-1-yl]carbonyl}-2-piperazinone, AC-35939, AS-10121, DA-41729, SY283510, SY289992, DB-003450, DB-015048, CS-0007767, FT-0700329, FT-0713388, FT-0771774, FT-0773561, SW220144-1, H10266, A861337, J-514247, J-523776, BRD-A12230535-001-01-8, Q27165473, Z2159890360, (+-)-4-[4,5-dihydro-imidazole-1-carbonyl]piperazin-2-one, (-)-4-(4,5-bis-(4-Chlorophenyl)-2-(2-isopropoxy-4-methoxyphenyl)-4,5-dihydroimidazole-1-carbonyl)piperazine-2-one, (??)-4-[4,5-Bis(4-chlorophenyl)-2-(2-isopropoxy-4-methoxy-phenyl)-4,5-dihydro-imidazole-1-carbonyl]-piperazin-2-one, 4-{[(4S,5R)-4,5-Bis(4-chlorophenyl)-2-(2-isopropoxy-4-methoxyphenyl)-4,5-dihydro-1H-imidazol-1-yl]carbonyl}-2-piperazinone, RG7112;R7112;rel-4-((4R,5S)-4,5-bis(4-chlorophenyl)-2-(2-isopropoxy-4-methoxyphenyl)-4,5-dihydro-1H-imidazole-1-carbonyl)piperazin-2-one