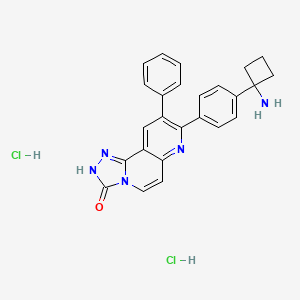
1032350-13-2, MK-2206 dihydrochloride, MK-2206 2HCl, MK2206, 8-(4-(1-Aminocyclobutyl)phenyl)-9-phenyl-[1,2,4]triazolo[3,4-f][1,6]naphthyridin-3(2H)-one dihydrochloride, 8-[4-(1-AMINOCYCLOBUTYL)PHENYL]-9-PHENYL-1,2,4-TRIAZOLO[3,4-F][1,6]NAPHTHYRIDIN-3(2H)-ONE DIHYDROCHLORIDE, Q34I3E28IO, 8-[4-(1-aminocyclobutyl)phenyl]-9-phenyl-2H-[1,2,4]triazolo[3,4-f][1,6]naphthyridin-3-one;dihydrochloride, 1,2,4-Triazolo(3,4-f)(1,6)naphthyridin-3(2H)-one, 8-(4-(1-aminocyclobutyl)phenyl)-9-phenyl-, hydrochloride (1:2), MK-2206 HCl salt, UNII-Q34I3E28IO, C25H21N5O.2HCl, AHU377 calcium salt, MK 2206 dihydrochloride, C25H23Cl2N5O, CHEMBL4635254, SCHEMBL17100521, EX-A259, HB5934, MFCD14584463, s1078, AKOS015966903, CCG-264809, PB19401, 8-[4-(1-aminocyclobutyl)phenyl]-9-phenyl-2H-[1,2,4]triazolo[3,4-f][1,6]naphthyridin-3-one;dihydrochl, AC-28437, AS-16298, FT-0672430, SW202557-3, P11738, J-000912, J-519356, Q27286944, (2R,4S)-4-[(3-Carboxy-1-oxopropyl)amino]-4-[(p-phenylphenyl)methyl]-2-methylbutanoic acid ethyl ester, 4-[[(2S,4R)-5-Ethoxy-4-methyl-5-oxo-1-(4-phenylphenyl)pentan-2-yl]amino]-4-oxobutanoic acid calcium salt, 4-{[(2S,4R)-1-(4-Biphenylyl)-5-ethoxy-4-methyl-5-oxo-2-pentanyl]amino}-4-oxobutanoic acid, 8-[4-(1-Aminocyclobutyl)phenyl]-9-phenyl-1,2,4-triazolo[3,4-f][1,6]naphthyridin-3(2H)-one 2HCl