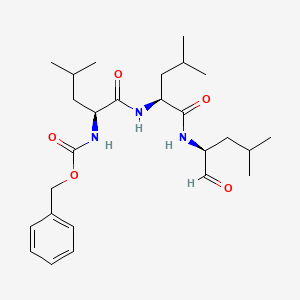
MG-132, 133407-82-6, Z-Leu-leu-leu-al, MG132, MG 132, Zlllal, Z-Leu-leu-leucinal, Z-LLL-CHO, Zlll-cho, Z-Leu-Leu-Leu-H, Carbobenzoxy-leucyl-leucyl-leucinal, C26H41N3O5, Benzyl ((S)-4-methyl-1-(((S)-4-methyl-1-(((S)-4-methyl-1-oxopentan-2-yl)amino)-1-oxopentan-2-yl)amino)-1-oxopentan-2-yl)carbamate, Benzyloxycarbonylleucyl-leucyl-leucine aldehyde, Benzyloxycarbonyl-leu-leu-leu-aldehyde, Benzyloxycarbonyl-leucyl-leucyl-leucinal, Carbobenzoxyl-leucinyl-leucinyl-leucinal-H, Cbz-Leu-Leu-Leu-H, benzyl (S)-4-methyl-1-((S)-4-methyl-1-((S)-4-methyl-1-oxopentan-2-ylamino)-1-oxopentan-2-ylamino)-1-oxopentan-2-ylcarbamate, RF1P63GW3K, CHEMBL64925, Cbz-L-Leu-L-Leu-L-Leu-CHO, CHEBI:75142, MFCD00674886, Lll cpd, (S)-N-((Phenylmethoxy)carbonyl)-L-leucyl-N-(1-formyl-3-methylbutyl)-L-leucinamide, N-[(Benzyloxy)carbonyl]-L-Leucyl-N-[(2s)-4-Methyl-1-Oxopentan-2-Yl]-L-Leucinamide, N-[(Phenylmethoxy)carbonyl]-L-leucyl-N-[(1S)-1-formyl-3-methylbutyl]-L-leucinamide, BRD0970, BRD-0970, Z-LLL, (S)-MG132, n-benzyloxycarbonyl-l-leucyl-l-leucyl-l-leucinal, UNII-RF1P63GW3K, Carbobenzoxy-L-leucyl-L-leucyl-L-leucinal, N-[(benzyloxy)carbonyl]-L-leucyl-N-[(1S)-1-formyl-3-methylbutyl]-L-leucinamide, zLLL, 1211877-36-9, Benzyl N-[(1S)-3-methyl-1-[[(1S)-3-methyl-1-[[(2S)-4-methyl-1-oxo-pentan-2-yl]carbamoyl]butyl]carbamoyl]butyl]carbamate, Z-LLLal, Cbz-Leu-Leu-Leucinal, Z-LLL-H, Z-L-leu-L-leu-L-leu-H, BSPBio_001310, carbobenzoxy-Leu-Leu-leucinal, KBioGR_000030, KBioSS_000030, MLS006011220, Cbz-L-Leu-L-Leu-L-Leu-H, SCHEMBL160925, GTPL8616, DTXSID3042639, BCBcMAP01_000028, KBio2_000030, KBio2_002598, KBio2_005166, KBio3_000059, KBio3_000060, benzyloxycarbonyl-Leu-Leu-leucinal, Bio2_000030, Bio2_000510, GLXC-03835, HMS1361B12, HMS1791B12, HMS1989B12, HMS3402B12, AMY40914, EX-A1500, BDBM50069985, HB4135, NSC782153, PI-102, s2619, AKOS027420457, MG-132 [Z-Leu- Leu-Leu-CHO], CCG-207860, CCG-208036, CS-0471, MG-132?, NSC-782153, compound 5b [PMID: 16686537], IDI1_033780, s10322, NCGC00161679-01, NCGC00161679-02, NCGC00161679-03, NCGC00161679-04, NCGC00161679-13, Z-Leu-Leu-Leu-al, >=90% (HPLC), AS-55854, benzyl N-[(1S)-3-methyl-1-{[(1S)-3-methyl-1-{[(2S)-4-methyl-1-oxopentan-2-yl]carbamoyl}butyl]carbamoyl}butyl]carbamate, HY-13259, SMR002530629, SW219780-1, UNM000011053701, Z-LLL-al , Z-Leu-Leu-Leu-CHO, A806612, Q3272916, SR-01000864598-1, BRD-K60230970-001-04-3, BRD-K60230970-001-05-0, BRD-K60230970-001-06-8, BRD-K60230970-001-07-6, BRD-K60230970-001-08-4, BRD-K60230970-001-10-0, MG-132 - CAS 133407-82-6, MG-132, >/=95% by HPLC - CAS 133407-82-6, L-Leucinamide, N-((phenylmethoxy)carbonyl)-L-leucyl-N-((1S)-1-formyl-3-methylbutyl)-, L-Leucinamide, N-((phenylmethoxy)carbonyl)-L-leucyl-N-(1-formyl-3-methylbutyl)-, (S)-, L-Leucinamide, N-[(phenylmethoxy)carbonyl]-L-leucyl-N1-[(1S)-1-formyl-3-methylbutyl]-, (S)-4-methyl-2-(3-phenyl-propionylamino)-pentanoic acid [(S)-1-((S)-1-formyl-3-methyl-butylcarbamoyl)-3-methyl-butyl]-amide, {(S)-1-[(S)-1-((S)-1-Formyl-3-methyl-butylcarbamoyl)-3-methyl-butylcarbamoyl]-3-methyl-butyl}-carbamic acid benzyl ester, {1-[(S)-(S)-1-((S)-1-Formyl-3-methyl-butylcarbamoyl)-3-methyl-butylcarbamoyl]-3-methyl-butyl}-carbamic acid benzyl ester, {1-[1-(1-Formyl-3-methyl-butylcarbamoyl)-3-methyl-butylcarbamoyl]-3-methyl-butyl}-carbamic acid benzyl ester, benzyl N-[(1S)-1-[[(1S)-1-[[(1S)-1-formyl-3-methyl-butyl]carbamoyl]-3-methyl-butyl]carbamoyl]-3-methyl-butyl]carbamate, benzyl(S)-4-methyl-1-((S)-4-methyl-1-((S)-4-methyl-1-oxopentan-2-ylamino)-1-oxopentan-2-ylamino)-1-oxopentan-2-ylcarbamate, phenylmethyl N-[(2S)-4-methyl-1-[[(2S)-4-methyl-1-[[(2S)-4-methyl-1-oxopentan-2-yl]amino]-1-oxopentan-2-yl]amino]-1-oxopentan-2-yl]carbamate