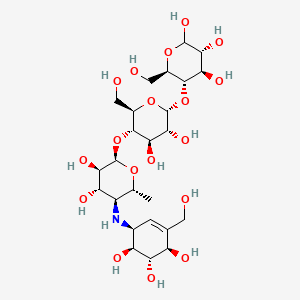
acarbose, Glucobay, CHEBI:2376, Bay g 5421, MFCD00869592, Acarbose hydrate, BAY-g 5421, (3R,4R,5S,6R)-5-{[(2R,3R,4R,5S,6R)-5-{[(2R,3R,4S,5S,6R)-3,4-dihydroxy-6-methyl-5-{[(1S,4R,5S,6S)-4,5,6-trihydroxy-3-(hydroxymethyl)cyclohex-2-en-1-yl]amino}oxan-2-yl]oxy}-3,4-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-6-(hydroxymethyl)oxane-2,3,4-triol, 4,6-dideoxy-4-{[(1S,4R,5S,6S)-4,5,6-trihydroxy-3-(hydroxymethyl)cyclohex-2-en-1-yl]amino}-alpha-D-glucopyranosyl-(1->4)-alpha-D-glucopyranosyl-(1->4)-D-glucopyranose, SMR000466376, SR-01000759407, Glucobay;, Prandase;, Precose;, Acarbose,(S), Acarbose; O-4,6-Dideoxy-4-[[(1S,4R,5S,6S)-4,5,6-trihydroxy-3-(hydroxymethyl)cyclohex-2-enyl]amino]-a-d-glucopyranosyl-(1?4)-O-a-d-glucopyranosyl-(1?4)-d-glucopyranose, Precose (TN), Acarbose for identification, Acarbose (JAN/USAN/INN), MLS000759506, MLS001424056, MLS006011898, SPECTRUM1505172, CHEMBL404271, SCHEMBL5316305, Acarbose for peak identification, CHEMBL3734896, BDBM23406, XUFXOAAUWZOOIT-UGEKTDRHSA-N, HMS2051F03, HMS2093I22, HMS2236P06, Pharmakon1600-01505172, C25H43NO18;, BBL030515, BDBM50180587, NSC758915, STK801930, AKOS005622515, CCG-100913, CCG-213345, MD-0230, NC00163, NCGC00160515-01, (3R,4R,5S,6R)-5-[(2R,3R,4R,5S,6R)-5-[(2R,3R,4S,5S,6R)-3,4-dihydroxy-6-methyl-5-[[(1S,4R,5S,6S)-4,5,6-trihydroxy-3-(hydroxymethyl)cyclohex-2-en-1-yl]amino]oxan-2-yl]oxy-3,4-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-6-(hydroxymethyl)oxane-2,3,4-triol, SBI-0206777.P001, C06802, D00216, AB00639959-06, AB00639959_08, Abamectin, Antibiotic for Culture Media Use Only, Q-200574, SR-01000759407-4, SR-01000759407-5, SR-01000759407-6, BRD-A16444946-001-07-1, 4",6"-Dideoxy-4"-([1S]-[1,4,6/5]-4,5,6-trihydroxy-3-hydroxymethyl-2-yclohexenylamino)-maltotriose, 4,6-dideoxy-4-{[(1S,4R,5S,6S)-4,5,6-trihydroxy-3-(hydroxymethyl)cyclohex-2-en-1-yl]amino}-alpha-D-glucopyranosyl-(1->4)-alpha-D-glucopyranosyl-(1->4)-D-glucopyranose; Precose (TN);