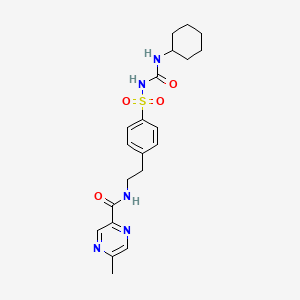
glipizide, 29094-61-9, Glucotrol, Glydiazinamide, Dipazide, Glibenese, Glucozide, Glupizide, Melizide, Minodiab, Napizide, Sucrazide, Mindiab, Glucotrol XL, Glibetin, Glidiab, Glucolip, Glupitel, Minidab, Minidiab, Aldiab, Digrin, Glican, Glipid, Glyde, Ozidia, Gluco-Rite, Glipizidum, Glipizida, Glipizidum [INN-Latin], Glipizida [INN-Spanish], K 4024, N-(4-(N-(cyclohexylcarbamoyl)sulfamoyl)phenethyl)-5-methylpyrazine-2-carboxamide, CP 28720, CP-28720, K-4024, TK 1320, CP 28,720, Glipizide slow release, CP-28,720, UNII-X7WDT95N5C, EINECS 249-427-6, X7WDT95N5C, MFCD00072159, 1-Cyclohexyl-3-((p-(2-(5-methylpyrazinecarboxamido)ethyl)phenyl)sulfonyl)urea, NSC-759120, BRN 0903495, CHEBI:5384, DTXSID0040676, N-[2-[4-(cyclohexylcarbamoylsulfamoyl)phenyl]ethyl]-5-methylpyrazine-2-carboxamide, Glidiazinamide, Glypidizine, Pyrazinecarboxamide, N-(2-(4-((((cyclohexylamino)carbonyl)amino)sulfonyl)phenyl)ethyl)-5-methyl-, Glide, CHEMBL1073, MLS000069386, N-(4-(beta-(5-Methylpyrazine-2-carboxamido)ethyl)benzenesulphonyl)-N'-cyclohexylurea, CP 28720;K 4024, DTXCID8020676, Glipizide [USAN:USP:INN:BAN], METAGLIP COMPONENT GLIPIZIDE, Urea, 1-cyclohexyl-3-((p-(2-(5-methylpyrazinecarboxamido)ethyl)phenyl)sulfonyl)-, N-(2-{4-[(cyclohexylcarbamoyl)sulfamoyl]phenyl}ethyl)-5-methylpyrazine-2-carboxamide, N-[2-(4-{[(cyclohexylcarbamoyl)amino]sulfonyl}phenyl)ethyl]-5-methylpyrazine-2-carboxamide, NSC 759120, NCGC00015462-07, SMR000058455, Glipizidum (INN-Latin), CAS-29094-61-9, Glipizida (INN-Spanish), GLIPIZIDE (MART.), GLIPIZIDE [MART.], 1-cyclohexyl-3-[[p-[2-(5-methylpyrazinecarboxamido)ethyl]phenyl]sulfonyl]urea, 1-Cyclohexyl-3-{4-[2-(5-methylpyrazine-2-carboxamido)ethyl]phenylsulfonyl}urea, GLIPIZIDE (USP-RS), GLIPIZIDE [USP-RS], GLIPIZIDE (EP MONOGRAPH), GLIPIZIDE [EP MONOGRAPH], GLIPIZIDE (USP MONOGRAPH), GLIPIZIDE [USP MONOGRAPH], Glipizide (USAN:USP:INN:BAN), 1-cyclohexyl-3-({p-[2-(5-methylpyrazinecarboxamido)ethyl]phenyl}sulfonyl)urea, N-{4-[beta-(5-methylpyrazine-2-carboxamido)ethyl]benzenesulphonyl}-N'-cyclohexylurea, Glucotrol (TN), Pyrazinecarboxamide, N-[2-[4-[[[(cyclohexylamino)carbonyl]amino]sulfonyl]phenyl]ethyl]-5-methyl-, Glipizide (USP/INN), SR-01000000010, Glipizideer, GlipizideXL, GlucotrolXL, GlipizideERER, Glipizide ER, Glipizide XL, Glipizide, solid, N-(2-(4-((cyclohexylcarbamoyl)sulfamoyl)phenyl)ethyl)-5-methylpyrazine-2-carboxamide, N-(4-[N-(cyclohexylcarbamoyl)sulfamoyl]phenethyl)-5-methylpyrazine-2-carboxamide, N-[4-[N-(Cyclohexylcarbamoyl)sulfamoyl]phenethyl]-5-methylpyrazine-2-carboxamide, glipizide/glucotrol, Glipizide,(S), N-[2-[4[[[(Cyclohexylamino)carbonyl]amino]sulfonyl]phenyl]ethyl]-5-methylpyrazinecarboxamide; 1-Cyclohexyl-3-[[p-[2-(5-methylpyrazinecarboxamido), Prestwick_242, KS-1068, Metaglip (Salt/Mix), Glipizide (Glucotrol), GLIPIZIDE [INN], GLIPIZIDE [JAN], GLIPIZIDE [MI], Lopac-G-117, GLIPIZIDE [USAN], Opera_ID_1908, Prestwick0_000131, Prestwick1_000131, Prestwick2_000131, Prestwick3_000131, GLIPIZIDE [VANDF], G-117, GLIPIZIDE [WHO-DD], CBiol_001788, Lopac0_000621, SCHEMBL17094, BSPBio_000202, BSPBio_001349, KBioGR_000069, KBioSS_000069, MLS001148176, BIDD:GT0476, SPBio_002141, BPBio1_000224, GTPL6821, GLIPIZIDE [ORANGE BOOK], KBio2_000069, KBio2_002637, KBio2_005205, KBio3_000137, KBio3_000138, Bio1_000074, Bio1_000563, Bio1_001052, Bio2_000069, Bio2_000549, GLXC-20257, HMS1361D11, HMS1568K04, HMS1791D11, HMS1989D11, HMS2089C21, HMS2093J09, HMS2095K04, HMS2233N11, HMS3259K12, HMS3261N04, HMS3369L12, HMS3402D11, HMS3655G04, HMS3712K04, Pharmakon1600-01505433, BCP09195, HY-B0254, Tox21_110156, Tox21_301834, Tox21_500621, BBL028143, BDBM50012956, NSC759120, NSC813218, s1715, STK631952, AKOS005564405, GLIPIZIDE COMPONENT OF METAGLIP, N-[4-(3-Cyclohexylureidosulfonyl)phenethyl]-5-methyl-2-pyrazinecarboxamide, Tox21_110156_1, CCG-204710, DB01067, LP00621, NC00564, NSC-813218, SDCCGSBI-0050603.P003, IDI1_033819, Glipizide 100 microg/mL in Acetonitrile, NCGC00015462-01, NCGC00015462-02, NCGC00015462-03, NCGC00015462-04, NCGC00015462-05, NCGC00015462-06, NCGC00015462-08, NCGC00015462-09, NCGC00015462-10, NCGC00015462-11, NCGC00015462-12, NCGC00015462-14, NCGC00015462-23, NCGC00016802-01, NCGC00023748-03, NCGC00023748-04, NCGC00023748-05, NCGC00023748-06, NCGC00023748-07, NCGC00255522-01, NCGC00261306-01, 2-Pyrazinecarboxamide, N-[2-[4-[[[(cyclohexylamino)carbonyl]amino]sulfonyl]phenyl]ethyl]-5-methyl-, AC-15789, SY009252, SBI-0050603.P002, EU-0100621, FT-0626714, FT-0659737, G0369, NS00009815, SW196549-3, D00335, Q3108899, SR-01000000010-2, SR-01000000010-5, W-107005, BRD-K12219985-001-04-8, BRD-K12219985-001-15-4, Z1575081877, Glipizide, European Pharmacopoeia (EP) Reference Standard, Glipizide, United States Pharmacopeia (USP) Reference Standard, Glipizide, Pharmaceutical Secondary Standard; Certified Reference Material, 1-cyclohexyl-3-(4-(2-(2-methylpyrazine-5-carboxamido)ethyl)phenylsulfonyl)urea, 1-Cyclohexyl-3-{{p=[2-[5-methylpyrazine-carboxamido)-ethyl]phenyl}sulphonyl}urea, N-(4-(.beta.-(5-Methylpyrazine-2-carboxamido)ethyl)benzenesulphonyl)-N'-cyclohexylurea, 172964-66-8, N-(2-[4-(([(Cyclohexylamino)carbonyl]amino)sulfonyl)phenyl]ethyl)-5-methyl-2-pyrazinecarboxamide