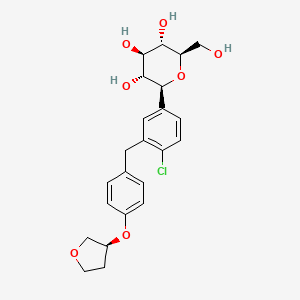
Empagliflozin, 864070-44-0, JARDIANCE, BI 10773, BI10773, BI-10773, Empagliflozin (BI 10773), UNII-HDC1R2M35U, HDC1R2M35U, (2S,3R,4R,5S,6R)-2-(4-chloro-3-(4-(((S)-tetrahydrofuran-3-yl)oxy)benzyl)phenyl)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol, CHEBI:82720, 1-chloro-4-(glucopyranos-1-yl)-2-(4-(tetrahydrofuran-3-yloxy)benzyl)benzene, (2S,3R,4R,5S,6R)-2-[4-chloro-3-({4-[(3S)-oxolan-3-yloxy]phenyl}methyl)phenyl]-6-(hydroxymethyl)oxane-3,4,5-triol, (1S)-1,5-anhydro-1-(4-chloro-3-{4-[(3S)-tetrahydrofuran-3-yloxy]benzyl}phenyl)-D-glucitol, GLYXAMBI COMPONENT EMPAGLIFLOZIN, (1S)-1,5-Anhydro-1-C-[4-chloro-3-[[4-[[(3S)-tetrahydro-3-furanyl]oxy]phenyl]methyl]phenyl]-D-glucitol, TRIJARDY XR COMPONENT EMPAGLIFLOZIN, (2S,3R,4R,5S,6R)-2-[4-CHLORO-3-[[4-[(3S)-OXOLAN-3-YL]OXYPHENYL]METHYL]PHENYL]-6-(HYDROXYMETHYL)OXANE-3,4,5-TRIOL, D-Glucitol, 1,5-anhydro-1-C-(4-chloro-3-((4-(((3S)-tetrahydro-3-furanyl)oxy)phenyl)methyl)phenyl)-, (1S)-, Empagliflozin (BI-10773;BI 10773;BI10773), (1S)-1,5-ANHYDRO-1-C-(4-CHLORO-3-((4-(((3S)-OXAN-3-YL)OXY)PHENYL)METHYL)PHENYL)-D-GLUCITOL, (1S)-1,5-anhydro-1-C-{4-chloro-3-((4-{((3S)-oxolan-3-yl)oxy}phenyl)methyl)phenyl}-D-glucitol, (2S,3R,4R,5S,6R)-2-[4-chloranyl-3-[[4-[(3S)-oxolan-3-yl]oxyphenyl]methyl]phenyl]-6-(hydroxymethyl)oxane-3,4,5-triol, Empagliflozin [INN], Empagliflozin [USAN:INN], Empagliflozina, Empagliflozine, Empagliflozinum, C23H27ClO7, MFCD22566222, Jardiance (TN), (1S)-1,5-anhydro-1-(4-chloro-3-(4-((3S)-tetrahydrofuran-3-yloxy)benzyl)phenyl)-D-glucitol, (1S)-1,5-Anhydro-1-C-(4-chloro-3-((4-(((3S)-oxolan-3-yl)oxy)phenyl)methyl)phenyl)-D-glucitol, 7R3, D-Glucitol, 1,5-anhydro-1-C-[4-chloro-3-[[4-[[(3S)-tetrahydro-3-furanyl]oxy]phenyl]methyl]phenyl]-, (1S)-; (1S)-1,5-Anhydro-1-C-[4-chloro-3-[[4-[[(3S)-tetrahydro-3-furanyl]oxy]phenyl]methyl]phenyl]-D-glucitol; BI 10773; Empagliflozin; Jardiance, EMPAGLIFLOZIN [MI], BI-10773;Empagliflozin, EMPAGLIFLOZIN [JAN], EMPAGLIFLOZIN [USAN], Empagliflozin (BI10773), EMPAGLIFLOZIN [VANDF], SCHEMBL899986, EMPAGLIFLOZIN [WHO-DD], GTPL4754, CHEMBL2107830, Empagliflozin (JAN/USAN/INN), A10BK03, AMY1858, EX-A414, BDBM150162, DTXSID601026093, EMPAGLIFLOZIN [ORANGE BOOK], BBL104150, HB4638, s8022, STL557964, US8980829, EMPAGLIFLOZIN, AKOS024464680, CCG-269242, CS-0940, DB09038, DS-9824, PB23119, AC-27643, EMPAGLIFLOZIN COMPONENT OF GLYXAMBI, EMPAGLIFLOZIN COMPONENT OF SYNJARDY, HY-15409, EMPAGLIFLOZIN COMPONENT OF TRIJARDY XR, SW219120-1, C22194, D10459, EN300-7422890, A852380, AU-004/43508285, Q5373824, Z2235802079, 1,5-anhydro-1-{4-chloro-3-[4-(tetrahydro-3-furanyloxy)benzyl]phenyl}hexitol, (2S,3R,4R,5S,6R)-2-(4-chloro-3-(4-((S)-tetrahydrofuran-3-yloxy)benzyl)phenyl)-6-(hydroxyMethyl)-tetrahydro-2H-pyran-3,4,5-triol, (2S,3R,4R,5S,6R)-2-(4-Chloro-3-(4-((S)-tetrahydrofuran-3-yloxy)benzyl)phenyl)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol, (2S,3R,4R,5S,6R)-2-[4-Chloro-3-[[4-[(3S)-tetrahydrofuran-3-yl]oxyphenyl]methyl]phenyl]-6-(hydroxymethyl)tetrahydropyran-3,4,5-triol