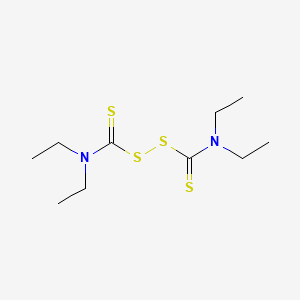
disulfiram, Tetraethylthiuram disulfide, 97-77-8, Antabuse, Bis(diethylthiocarbamoyl) disulfide, Antabus, Alcophobin, Anticol, Esperal, Teturam, TETD, Dicupral, Exhorran, Hoca, Ethyldithiurame, Abstensil, Antaethyl, Antietanol, Antivitium, Contralin, Tetradine, Tetraetil, Teturamin, Abstinil, Abstinyl, Antadix, Antalcol, Antetan, Antetil, Antietil, Antikol, Aversan, Averzan, Cronetal, Krotenal, Refusal, Etabus, Ethyl tuads, Ethyl Thiram, Ethyl Thiurad, Ethyl Tuex, Antaenyl, Antaetil, Antiaethan, Contrapot, Disulfan, Disulfuram, Ephorran, Stopetyl, Thiuranide, Anteyl, Bonibal, Disetil, Nocbin, Tenurid, Tenutex, Tetidis, Ekagom TEDS, Ekagom TETDS, Ethyldithiourame, Noxal, Anti-ethyl, Alk-aubs, Tetraethylthiuram disulphide, Thiuram E, TATD, Soxinol TET, Tetraethylthiram disulfide, Ekagom DTET, Accel TET, Espenal, Exhoran, Sanceler TET-G, Ro-sulfiram, Tetraethylthioperoxydicarbonic diamide, Tetraethylthiuram, Tuads, ethyl, Usaf B-33, Sanceler TET, Stopaethyl, Thireranide, Antaethan, Antethyl, Tetradin, Tillram, Accel TET-R, Ethyl Thiudad, Dupon 4472, Tetraethylthiuran disulfide, Anthethyl, Disulphuram, Dupont fungicide 4472, Hocakrotenalnci-C02959, Tetraethylthiram disulphide, Bis(diethylthiocarbamyl) disulfide, Tetraethylthiuram sulfide, Thiuram disulfide, tetraethyl-, N,N,N',N'-Tetraethylthiuram disulfide, Antabuse (TN), Bis(N,N-diethylthiocarbamoyl) disulfide, 1,1'-Dithiobis(N,N-diethylthioformamide), Disulfide, bis(diethylthiocarbamoyl), ENT 27,340, Thioperoxydicarbonic diamide, tetraethyl-, Bis(diethylthiocarbamoyl)disulphide, NCI-C02959, diethylcarbamothioylsulfanyl N,N-diethylcarbamodithioate, NSC-25953, N,N-diethyl[(diethylcarbamothioyl)disulfanyl]carbothioamide, NSC 190940, Bis(N,N-diethylthiocarbamoyl)disulphide, N,N,N',N'-Tetraethylthiuram disulphide, Bis((diethylamino)thioxomethyl)disulphide, Bis((diethylamino)thioxomethyl) disulfide, Tetraethylthiuram disulfide;TETD, TTS, TR3MLJ1UAI, MFCD00009048, Thioperoxydicarbonic diamide ([(H2N)C(S)]2S2), tetraethyl-, CHEMBL964, MLS000069818, CHEBI:4659, ORA102, DTXSID1021322, ORA-102, NSC25953, Esperal [France], CAS-97-77-8, NCGC00016000-08, NCGC00016000-13, SMR000059171, Thioperoxydicarbonic diamide (((H2N)C(S))2S2), tetraethyl-, 1,1',1'',1'''-[disulfanediylbis(carbonothioylnitrilo)]tetraethane, Ancazide ET, Akrochem TETD, Perkacit TETD, Ekaland TETD, Perkait TETD, Ethyl Tuads Rodform, C10H20N2S4, DTXCID101322, Disulfiramum [INN-Latin], Disulfiramo [INN-Spanish], Bis[(diethylamino)thioxomethyl] disulfide, CCRIS 582, 1,1'-Dithiobis[N,N-diethylthioformamide], HSDB 3317, SR-01000076145, UNII-TR3MLJ1UAI, EINECS 202-607-8, NSC 25953, AI3-27340, Formamide, 1,1'-dithiobis(N,N-diethylthio-, Thioperoxydicarbonic diamide (((H2N)C(S))2S2), N,N,N',N'-tetraethyl-, Thioperoxydicarbonic diamide ([(H2N)C(S)]2S2), N,N,N',N'-tetraethyl-, Disulfiram [USP:INN:BAN:JAN], Bis(diethylthiocarbamyoyl)disulfide, Prestwick_182, Disulfiram (Antabuse), Spectrum_001010, CPD000059171, Disulfiram (Antabuse)?, DISULFIRAM [MI], Opera_ID_224, DISULFIRAM [INN], DISULFIRAM [JAN], Prestwick0_000097, Prestwick1_000097, Prestwick2_000097, Prestwick3_000097, Spectrum2_001176, Spectrum3_000405, Spectrum4_000228, Spectrum5_001590, DISULFIRAM [HSDB], DISULFIRAM [IARC], Lopac-T-1132, 1,N-diethylthioformamide], DISULFIRAM [VANDF], Formamide, 1,1'-dithiobis(N,N-diethylthio)-, UPCMLD-DP090, EC 202-607-8, T 1132, tetraethyl thiuram disulfide, DISULFIRAM [MART.], Tetraethyldithiuram disulfide, DISULFIRAM [WHO-DD], Lopac0_001164, SCHEMBL27213, BSPBio_000054, BSPBio_001930, KBioGR_000895, KBioSS_001490, MLS000758264, MLS001076475, MLS001423963, SPECTRUM1500262, SPBio_001191, SPBio_001993, BPBio1_000060, Disulfiram (JP17/USP/INN), GTPL7168, DISULFIRAM [EP IMPURITY], DISULFIRAM [ORANGE BOOK], UPCMLD-DP090:001, KBio2_001490, KBio2_004058, KBio2_006626, KBio3_001150, DISULFIRAM [EP MONOGRAPH], Thioperoxydicarbonic diamide ((H2N)C(S))2S2, tetraethyl-, DISULFIRAM [USP MONOGRAPH], HMS1568C16, HMS1920I16, HMS2051M17, HMS2090C18, HMS2091O22, HMS2095C16, HMS2230K06, HMS3263J09, HMS3371B21, HMS3393M17, HMS3655I19, HMS3712C16, HMS3867H13, Pharmakon1600-01500262, BCP07331, HY-B0240, bis-(diethyl-thiocarbamyl)-disulfide, Tox21_110280, Tox21_300403, Tox21_400072, Tox21_501164, BDBM50058655, CCG-39549, DL-379, HB1119, N,N',N'-Tetraethylthiuram disulfide, NSC756748, NSC800739, s1680, STL069539, 1,1',1'',1'''-{disulfanediylbis[(thioxomethylene)-nitrilo]}tetraethane, AKOS000120201, Tox21_110280_1, AT13284, DB00822, HS-0057, LP01164, NC00063, NSC-756748, NSC-800739, SDCCGSBI-0051131.P005, WLN: 2N2 & YUS & S 2, NCGC00016000-01, NCGC00016000-02, NCGC00016000-03, NCGC00016000-04, NCGC00016000-05, NCGC00016000-06, NCGC00016000-07, NCGC00016000-09, NCGC00016000-10, NCGC00016000-11, NCGC00016000-12, NCGC00016000-14, NCGC00016000-15, NCGC00016000-18, NCGC00016000-29, NCGC00094423-01, NCGC00094423-02, NCGC00094423-03, NCGC00094423-05, NCGC00094423-06, NCGC00094423-07, NCGC00254447-01, NCGC00261849-01, N,N,N'',N''-tetraethylthiuram disulfide, BIS(DIETHYLTHIOCARBAMOYL) DISULPHIDE, Formamide,1'-dithiobis(N,N-diethylthio)-, SBI-0051131.P004, 1,1''-dithiobis(N,N-diethylthioformamide), DB-057683, AB00051976, B0479, EU-0101164, FT-0631502, FT-0667720, NS00010096, SW196492-4, Tetraethylthiuram disulfide, >=97.0% (S), EN300-19458, C01692, D00131, S00294, AB00051976-20, AB00051976-21, AB00051976-23, AB00051976_22, AB00051976_25, A845750, Q409665, Q-201812, SR-01000076145-1, SR-01000076145-5, SR-01000076145-8, BRD-K32744045-001-05-6, BRD-K32744045-001-17-1, Z104473910, Disulfiram, British Pharmacopoeia (BP) Reference Standard, Disulfiram, European Pharmacopoeia (EP) Reference Standard, Disulfiram, United States Pharmacopeia (USP) Reference Standard, 1,1'',1'''',1''''''-[disulfanediylbis(carbonothioylnitrilo)]tetraethane, Disulfiram, Pharmaceutical Secondary Standard; Certified Reference Material, N,N-diethylcarbamodithioic acid [[diethylamino(sulfanylidene)methyl]thio] ester, THIOPEROXYDICARBONIC DIAMIDE ((H2N)C(S))(SUB 2) S(SUB 2), TETRAETHYL-, InChI=1/C10H20N2S4/c1-5-11(6-2)9(13)15-16-10(14)12(7-3)8-4/h5-8H2,1-4H, TETRAETHYLTHIOPEROXYDICARBONIC DIAMIDE ((((C(SUB 2)H(SUB 5))(SUB 2)N)C(S))(SUB 2)S(SUB 2))