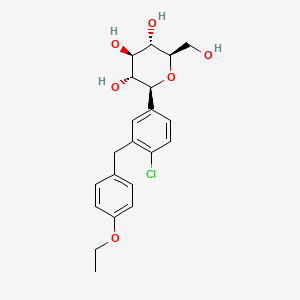
Dapagliflozin, 461432-26-8, BMS-512148, Forxiga, BMS 512148, (2S,3R,4R,5S,6R)-2-(4-chloro-3-(4-ethoxybenzyl)phenyl)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol, Dagagflozin, UNII-1ULL0QJ8UC, (1S)-1,5-Anhydro-1-C-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-D-glucitol, 1ULL0QJ8UC, CHEBI:85078, LYN-045, (2S,3R,4R,5S,6R)-2-{4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl}-6-(hydroxymethyl)oxane-3,4,5-triol, CHEMBL429910, DTXSID20905104, Dapagliflozin [USAN:INN], QTERNMET COMPONENT DAPAGLIFOZIN, DAPAGLIFLOZIN COMPONENT QTERNMET, (2S,3R,4R,5S,6R)-2-(4-Chloro-3-(4-ethoxybenzyl)phenyl)-6- (hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol, QTERNMET XR COMPONENT DAPAGLIFLOZIN, Forxiga (TN), D-Glucitol, 1,5-anhydro-1-C-(4-chloro-3-((4-ethoxyphenyl)methyl)phenyl)-, (1S)-, CHEMBL3125458, BMS512148, (2S,3R,4R,5S,6R)-2-[4-chloranyl-3-[(4-ethoxyphenyl)methyl]phenyl]-6-(hydroxymethyl)oxane-3,4,5-triol, (2S,3R,4R,5S,6R)-2-[4-Chloro-3-(4-ethoxybenzyl)phenyl]-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol, Dapagliflozin [USAN], dapagliflozine, dapagliflozinum, Dapagliflozina, (1S)-1,5-anhydro-1-(4-chloro-3-(4-ethoxybenzyl)phenyl)-D-glucitol, (1S)-1,5-anhydro-1-[4-chloro-3-(4-ethoxybenzyl)phenyl]-D-glucitol, (1S)-1,5-Anhydro-1-C-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-D-glucitol; (2S,3R,4R,5S,6R)-2-(4-Chloro-3-(4-ethoxybenzyl)phenyl)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol; (2S,3R,4R,5S,6R)-2-[4-Chloro-3-(4-ethoxybenzyl)phenyl]-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol; BMS 512148; Dapagliflozin; Farxiga, (2S,3R,4R,5S,6R)-2-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-6-(hydroxymethyl)oxane-3,4,5-triol, DAPAGLIFLOZIN?, S1548_Selleck, C-aryl glucoside, 6, DAPAGLIFLOZIN [MI], DAPAGLIFLOZIN [INN], Dapagliflozin (USAN/INN), 2-(3-(4-Ethoxybenzyl)-4-chlorophenyl)-6-hydroxymethyltetrahydro-2H-pyran-3,4,5-triol, DAPAGLIFLOZIN [VANDF], SCHEMBL157820, DAPAGLIFLOZIN [WHO-DD], GTPL4594, BR1019 component Dapagliflozin, BDBM20880, A10BK01, EX-A005, JVHXJTBJCFBINQ-ADAARDCZSA-N, BCPP000265, DTXCID101334207, DAPAGLIFLOZIN [ORANGE BOOK], AMY18541, BDBM50448923, MFCD13182359, s1548, AKOS005145763, BCP9000583, BL-0052, BMS5121458, CCG-229917, CS-0781, DB06292, EX-7214, NCGC00250402-09, AC-24699, BD164346, HY-10450, DAPAGLIFLOZIN COMPONENT OF QTERNMET XR, NS00099173, A25150, C22193, D08897, EN300-7407160, Q409898, J-500392, BRD-K58160573-001-01-3, BRD-K58160573-001-05-4, Z2235801883, (2S, 3R, 4R, 5S, 6R)-2-[4-chloro-3-(4-ethoxy-benzyl)-phenyl]-6-hydroxymethyl-tetrahydro-pyran-3,4,5-triol, (2S,3R,4R,5S,6R)-2-(3-(4-ethoxybenzyl)-4-chlorophenyl)-6-(hydroxymethyl)-tetrahydro-2H-pyran-3,4,5-triol, (2S,3R,4R,5S,6R)-2-(3-(4-ethoxybenzyl)-4-chlorophenyl)-6-(hydroxymethyl)-tetrahydro-2H-pyran-3,4,5-triol;BMS-512148, (2S,3R,4R,5S,6R)-2-[4-chloro-3-(4-ethoxy-benzyl)-phenyl]-6-hydroxymethyl-tetrahydro-pyran-3,4,5-triol, LE6