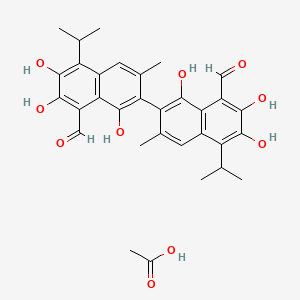
Gossypol acetic acid, 12542-36-8, gossypol-Acetic acid, Gossypol acetate, 866541-93-7, 5453-04-3, AT101, Gossypol (acetic acid), (S)-Gossypol (acetic acid), 1189561-66-7, (-)-Gossypol acetic acid, Gossypol acetate, (R)-, (R)-Gossypol acetic acid, Gossypol acetic acid, R-, Gossypol acetic acid, (R)-, R-(-)-gossypol acetic acid, Gossypol acetic acid clathrate, (R)-(-)-Gossypol acetic acid, Acetate gossypol, NSC 19048, U9GNI6VT5N, acetic acid;7-(8-formyl-1,6,7-trihydroxy-3-methyl-5-propan-2-ylnaphthalen-2-yl)-2,3,8-trihydroxy-6-methyl-4-propan-2-ylnaphthalene-1-carbaldehyde, (+/-)-Gossypol acetic acid, MLS000028630, MLS002702979, NSC19048, SMR000058743, 12542-36-8 (ACETIC ACID), NSC-19048, 115038-46-5, acetic acid compound with (S)-1,1',6,6',7,7'-hexahydroxy-5,5'-diisopropyl-3,3'-dimethyl-[2,2'-binaphthalene]-8,8'-dicarbaldehyde (1:1), (-)-GOSSYPOL; ACETIC ACID, (S)-Gossypol acetic acid, GOSSYPOLACETATE, (+/-)-Gossypol-acetic acid;BL 193 (acetic acid), UNII-U9GNI6VT5N, AT-101 (acetic acid), Acetate-gossypol, GossypolAcOHSalt, Aceticacidgossypol, Gossypol xAcetate, Gossypol AcOH Salt, AT 101 acetic acid, Opera_ID_1014, Gossypol acetic acid [MI], SCHEMBL352576, (R)-Gossypol acetic acid salt, CHEMBL1516388, Gossypol acetic acid [WHO-DD], HMS500I15, DTXSID90921593, HMS3651H13, BCP09006, BCP24040, CCG-39212, HY-15464A, MFCD00058385, NSC727858, s2303, s2812, AKOS022188380, AT-101 (AT101), CS-3859, NSC-727858, NCGC00178279-01, AC-34098, AS-15487, HY-17510, DB-081879, FT-0686636, FT-0768953, G0543, SW197103-3, F85115, F85296, ()-Gossypol-acetic acid;BL 193 (acetic acid), A900030, A920161, J-005228, 1,1',6,6',7,7'-Hexahydroxy-5,5'-diisopropyl-3,3'-dimethyl-[2,2'-binaphthalene]-8,8'-dicarbaldehyde acetate salt, 1,1',6,6',7,7'-Hexahydroxy-5,5'-diisopropyl-3,3'-dimethyl-2,2'-binaphthalene-8,8'-dicarbaldehyde - acetic acid (1:1), 732279-26-4, Acetic acid compound with 1,1',6,6',7,7'-hexahydroxy-5,5'-diisopropyl-3,3'-dimethyl-[2,2'-binaphthalene]-8,8'-dicarbaldehyde, Acetic acid compound with 1,1',6,6',7,7'-hexahydroxy-5,5'-diisopropyl-3,3'-dimethyl-[2,2'-binaphthalene]-8,8'-dicarbaldehyde (1:1), acetic acid compound with 1,1',6,6',7,7'-hexahydroxy-5,5'-diisopropyl-3,3'-dimethyl-2,2'-binaphthyl-8,8'-dicarbaldehyde (1:1), Acetic acid--1,1',6,6',7,7'-hexahydroxy-3,3'-dimethyl-5,5'-di(propan-2-yl)[2,2'-binaphthalene]-8,8'-dicarbaldehyde (1/1), aceticacid;7-(8-formyl-1,6,7-trihydroxy-3-methyl-5-propan-2-ylnaphthalen-2-yl)-2,3,8-trihydroxy-6-methyl-4-propan-2-ylnaphthalene-1-carbaldehyde, AT 101 acetic acid; AT101 acetic acid;AT-101 acetic acid; (-)-Gossypol acetic acid; (R)-Gossypol acetic acid; Gossypol acetic acid