NPs Basic Information
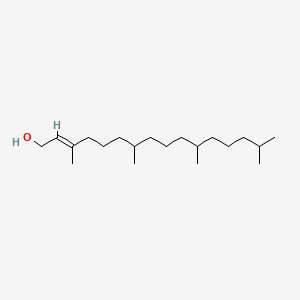
|
Name |
3,7,11,15-Tetramethyl-2-hexadecen-1-OL
|
| Molecular Formula | C20H40O | |
| IUPAC Name* |
(E)-3,7,11,15-tetramethylhexadec-2-en-1-ol
|
|
| SMILES |
CC(C)CCCC(C)CCCC(C)CCC/C(=C/CO)/C
|
|
| InChI |
InChI=1S/C20H40O/c1-17(2)9-6-10-18(3)11-7-12-19(4)13-8-14-20(5)15-16-21/h15,17-19,21H,6-14,16H2,1-5H3/b20-15+
|
|
| InChIKey |
BOTWFXYSPFMFNR-HMMYKYKNSA-N
|
|
| Synonyms |
Phytol; 3,7,11,15-TETRAMETHYL-2-HEXADECEN-1-OL; 7541-49-3; 3,7,11,15-Tetramethylhexadec-2-en-1-ol; 2-Hexadecen-1-ol, 3,7,11,15-tetramethyl-; (E)-3,7,11,15-tetramethylhexadec-2-en-1-ol; MFCD00151280; AI3-36488; Phytol, >=97%, FG; (2E)-3,7,11,15-tetramethylhexadec-2-en-1-ol; 3,7,11,15-teramethyl-2-hexadecene-1-ol-, (2E,7R,11R)-; 3,7,11,15-Tetramethyl-2-hexadecen-1-ol-, (2E,7R,11R)-; CHEMBL390773; AMY21944; s5121; AKOS008965402; CCG-267435; 253686-88-3; AS-47652; LS-14831; CS-0161167; 2-hexadecen-1-ol, 3,7,11,15-tetramethyl; EN300-16645; F14923; EN300-7399953; (2E)-3,7,11,15-Tetramethyl-2-hexadecen-1-ol; (E)-3,7,11,15-Tetramethyl-2-hexadecene-1-ol; Phytol 3,7,11,15-Tetramethyl-2-hexadecen-1-ol; W-108070; Z56347285; 3,7,11,15-Tetramethyl-2-hexadecen-1-ol (mixture of isomers); 4305EBEF-F02F-4CB0-985E-89469C273922
|
|
| CAS | 7541-49-3 | |
| PubChem CID | 5366244 | |
| ChEMBL ID | CHEMBL390773 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 296.5 | ALogp: | 8.2 |
| HBD: | 1 | HBA: | 1 |
| Rotatable Bonds: | 13 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 20.2 | Aromatic Rings: | 0 |
| Heavy Atoms: | 21 | QED Weighted: | 0.393 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.389 | MDCK Permeability: | 0.00001100 |
| Pgp-inhibitor: | 0.027 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.004 | 20% Bioavailability (F20%): | 0.605 |
| 30% Bioavailability (F30%): | 0.925 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.399 | Plasma Protein Binding (PPB): | 98.55% |
| Volume Distribution (VD): | 2.46 | Fu: | 2.06% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.348 | CYP1A2-substrate: | 0.19 |
| CYP2C19-inhibitor: | 0.327 | CYP2C19-substrate: | 0.097 |
| CYP2C9-inhibitor: | 0.444 | CYP2C9-substrate: | 0.947 |
| CYP2D6-inhibitor: | 0.113 | CYP2D6-substrate: | 0.047 |
| CYP3A4-inhibitor: | 0.182 | CYP3A4-substrate: | 0.122 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 6.197 | Half-life (T1/2): | 0.147 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.01 | Human Hepatotoxicity (H-HT): | 0.038 |
| Drug-inuced Liver Injury (DILI): | 0.037 | AMES Toxicity: | 0.001 |
| Rat Oral Acute Toxicity: | 0.008 | Maximum Recommended Daily Dose: | 0.154 |
| Skin Sensitization: | 0.969 | Carcinogencity: | 0.023 |
| Eye Corrosion: | 0.846 | Eye Irritation: | 0.932 |
| Respiratory Toxicity: | 0.06 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001818 | 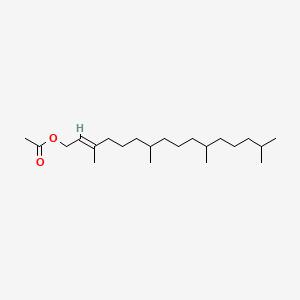 |
0.743 | D00FSV | 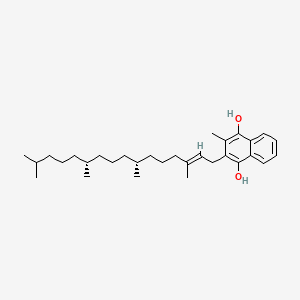 |
0.558 | ||
| ENC001412 | 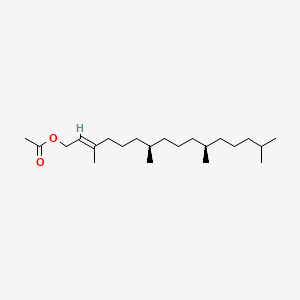 |
0.743 | D05XQE |  |
0.258 | ||
| ENC000766 |  |
0.689 | D0ZI4H |  |
0.225 | ||
| ENC000538 |  |
0.683 | D0D9NY |  |
0.224 | ||
| ENC000354 |  |
0.682 | D03LGY | 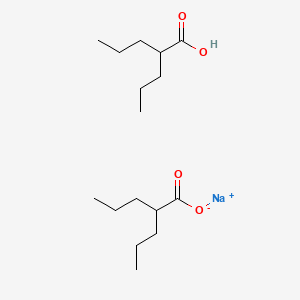 |
0.222 | ||
| ENC000902 |  |
0.667 | D0X4FM | 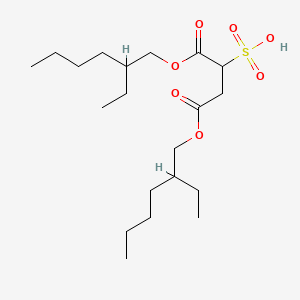 |
0.211 | ||
| ENC000537 |  |
0.639 | D05QNO |  |
0.207 | ||
| ENC000441 | 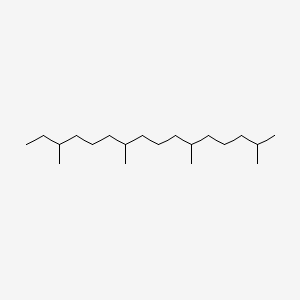 |
0.632 | D03JSJ |  |
0.205 | ||
| ENC001286 | 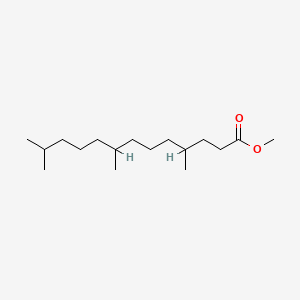 |
0.612 | D0N3NO |  |
0.202 | ||
| ENC000627 |  |
0.606 | D0T9TJ |  |
0.202 | ||