NPs Basic Information

|
Name |
(-)-Limonene
|
| Molecular Formula | C10H16 | |
| IUPAC Name* |
(4S)-1-methyl-4-prop-1-en-2-ylcyclohexene
|
|
| SMILES |
CC1=CC[C@H](CC1)C(=C)C
|
|
| InChI |
InChI=1S/C10H16/c1-8(2)10-6-4-9(3)5-7-10/h4,10H,1,5-7H2,2-3H3/t10-/m1/s1
|
|
| InChIKey |
XMGQYMWWDOXHJM-SNVBAGLBSA-N
|
|
| Synonyms |
(-)-Limonene; 5989-54-8; l-Limonene; (S)-Limonene; (S)-(-)-Limonene; (S)-1-Methyl-4-(prop-1-en-2-yl)cyclohex-1-ene; (4S)-limonene; S-(-)-Limonene; (-)-(4S)-Limonene; (S)-p-Mentha-1,8-diene; (-)-(S)-Limonene; (S)-(?)-Limonene; (4S)-1-methyl-4-(prop-1-en-2-yl)cyclohex-1-ene; Cyclohexene, 1-methyl-4-(1-methylethenyl)-, (4S)-; Limonene, L-; (-)-p-Mentha-1,8-diene; P-Mentha-1,8-diene, (S)-(-)-; Limonene, (-)-; (4S)-1-methyl-4-prop-1-en-2-ylcyclohexene; 47MAJ1Y2NE; CHEBI:15383; Cyclohexene, 1-methyl-4-(1-methylethenyl)-, (S)-; beta-Limonene; UNII-47MAJ1Y2NE; laevo-limonene; L-Limonen; EINECS 227-815-6; MFCD00001558; AI3-25390; EC 227-815-6; DSSTox_CID_27078; DSSTox_RID_82091; 1-Methyl-4-(1-methylethenyl)cyclohexene, (S)-; DSSTox_GSID_47078; 4alphaH-p-mentha-1,8-diene; (-)-LIMONENE [FCC]; CHEMBL236688; DTXSID6047078; (S)-(-)-Limonene, 96%; ZINC968226; (S)-(-)-p-mentha-1,8-diene; HY-Z0478; Tox21_302295; s6055; AKOS016844135; LMPR0102090002; (4S)-4-isopropenyl-1-methylcyclohexene; (S)-(-)-Limonene, analytical standard; ( inverted exclamation markA)-LIMONENE; (S)-(-)-Limonene, >=95%, FG; 4-Isopropenyl-1-methyl-1-cyclohexene #; NCGC00256073-01; AS-75559; CAS-5989-54-8; (4S)-1-methyl-4-isopropenylcyclohex-1-ene; CS-0014283; L0132; (S)-1-methyl-4-(1-methylethenyl)cyclohexene; C00521; EN300-196159; F20308; (4S)-1-Methyl-4-(prop-1-en-2-yl)cyclohexene; (4S)-1-methyl-4-(1-methyl ethenyl) cyclohexene; (S)-(-)-1-methyl-4-(1-methylethenyl)cyclohexene; W-110076; Q27089405; Z1255430958; (S)-(-)-Limonene, purum, >=95.0% (sum of enantiomers, GC); 9IU
|
|
| CAS | 5989-54-8 | |
| PubChem CID | 439250 | |
| ChEMBL ID | CHEMBL236688 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 136.23 | ALogp: | 3.4 |
| HBD: | 0 | HBA: | 0 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 0.0 | Aromatic Rings: | 1 |
| Heavy Atoms: | 10 | QED Weighted: | 0.477 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.371 | MDCK Permeability: | 0.00001890 |
| Pgp-inhibitor: | 0.007 | Pgp-substrate: | 0.003 |
| Human Intestinal Absorption (HIA): | 0.003 | 20% Bioavailability (F20%): | 0.94 |
| 30% Bioavailability (F30%): | 0.831 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.974 | Plasma Protein Binding (PPB): | 75.11% |
| Volume Distribution (VD): | 3.533 | Fu: | 19.97% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.703 | CYP1A2-substrate: | 0.564 |
| CYP2C19-inhibitor: | 0.252 | CYP2C19-substrate: | 0.775 |
| CYP2C9-inhibitor: | 0.055 | CYP2C9-substrate: | 0.774 |
| CYP2D6-inhibitor: | 0.02 | CYP2D6-substrate: | 0.851 |
| CYP3A4-inhibitor: | 0.06 | CYP3A4-substrate: | 0.252 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 13.285 | Half-life (T1/2): | 0.27 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.028 | Human Hepatotoxicity (H-HT): | 0.738 |
| Drug-inuced Liver Injury (DILI): | 0.057 | AMES Toxicity: | 0.015 |
| Rat Oral Acute Toxicity: | 0.022 | Maximum Recommended Daily Dose: | 0.785 |
| Skin Sensitization: | 0.846 | Carcinogencity: | 0.924 |
| Eye Corrosion: | 0.944 | Eye Irritation: | 0.981 |
| Respiratory Toxicity: | 0.448 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000555 |  |
1.000 | D0O1UZ | 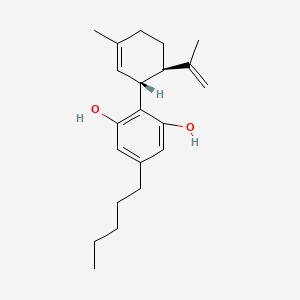 |
0.233 | ||
| ENC000369 | 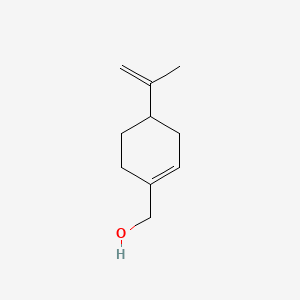 |
0.583 | D0A2AJ |  |
0.185 | ||
| ENC002276 | 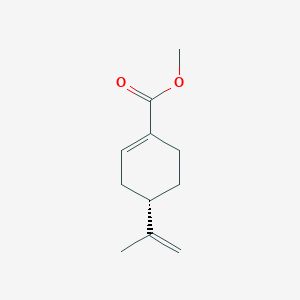 |
0.550 | D03KEK |  |
0.182 | ||
| ENC001981 |  |
0.545 | D0Z8SF |  |
0.174 | ||
| ENC002219 | 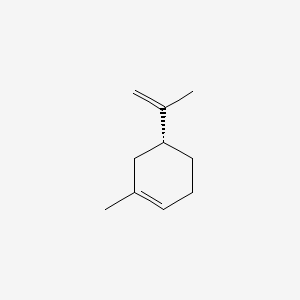 |
0.543 | D0WO8W |  |
0.167 | ||
| ENC002339 | 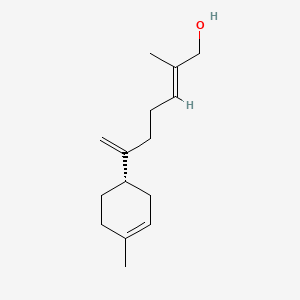 |
0.511 | D0B4RU |  |
0.167 | ||
| ENC001641 | 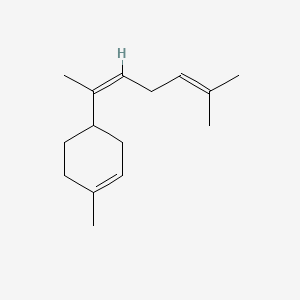 |
0.478 | D0H1QY | 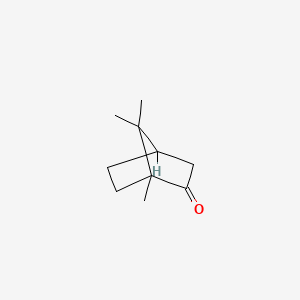 |
0.167 | ||
| ENC003255 | 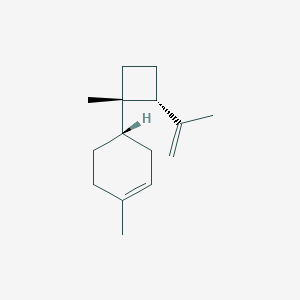 |
0.478 | D0K0EK | 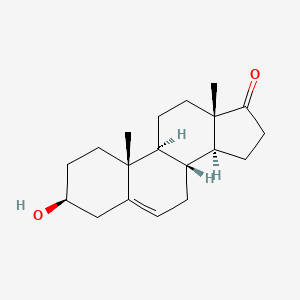 |
0.162 | ||
| ENC000194 | 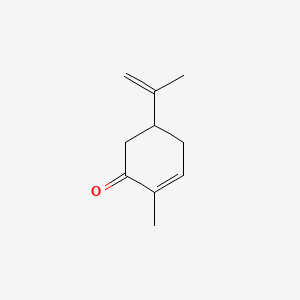 |
0.474 | D04GJN | 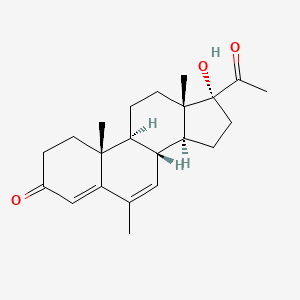 |
0.159 | ||
| ENC003150 | 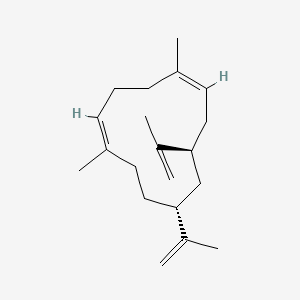 |
0.473 | D0RA9E | 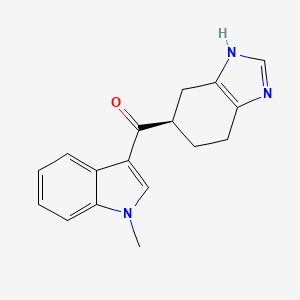 |
0.158 | ||