NPs Basic Information
|
Name |
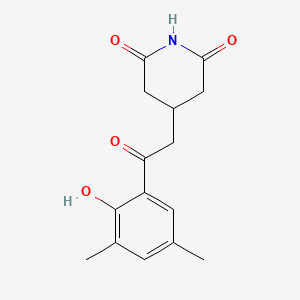
Actiphenol
|
| Molecular Formula | C15H17NO4 | |
| IUPAC Name* |
4-[2-(2-hydroxy-3,5-dimethylphenyl)-2-oxoethyl]piperidine-2,6-dione
|
|
| SMILES |
CC1=CC(=C(C(=C1)C(=O)CC2CC(=O)NC(=O)C2)O)C
|
|
| InChI |
InChI=1S/C15H17NO4/c1-8-3-9(2)15(20)11(4-8)12(17)5-10-6-13(18)16-14(19)7-10/h3-4,10,20H,5-7H2,1-2H3,(H,16,18,19)
|
|
| InChIKey |
YTLMIHBTPWTPEV-UHFFFAOYSA-N
|
|
| Synonyms |
ACTIPHENOL; Actinophenol; 526-02-3; Actiphenol [MI]; 3-(2-Hydroxy-3,5-dimethylphenacyl)glutarimide; NSC-58413; b-(3,5-dimethyl-2-hydroxybenzoylmethyl)glutarimide; MLS003559961; 1M5597X03D; SMR002227467; 4-[2-(2-hydroxy-3,5-dimethylphenyl)-2-oxoethyl]piperidine-2,6-dione; 2,6-Piperidinedione, 4-(2-(2-hydroxy-3,5-dimethylphenyl)-2-oxoethyl)-; 2,6-Piperidinedione, 4-[2-(2-hydroxy-3,5-dimethylphenyl)-2-oxoethyl]-; UNII-1M5597X03D; NSC58413; cid_245940; MEGxm0_000240; SCHEMBL3359715; CHEMBL3186963; ACon0_000590; ACon1_001537; Glutarimide,5-dimethylphenacyl)-; DTXSID50877820; CHEBI:181736; BDBM100273; ZINC1689064; Actiphenol, >=90% (LC/MS-ELSD); NCGC00180403-01; Glutarimide, 3-(2-hydroxy-3,5-dimethylphenacyl)-; BRD-K90243608-001-01-6; Q27252599; 2, 4-[2-(2-hydroxy-3,5-dimethylphenyl)-2-oxoethyl]-; 4-[2-(2-hydroxy-3,5-dimethyl-phenyl)-2-keto-ethyl]piperidine-2,6-quinone; 4-[2-(2-hydroxy-3,5-dimethyl-phenyl)-2-oxo-ethyl]piperidine-2,6-dione; 4-[2-(2-Hydroxy-3,5-dimethylphenyl)-2-oxoethyl]-2,6-piperidinedione #; 4-(2-(2-Hydroxy-3-(hydroxymethyl)-5-methylphenyl)-2-oxoethyl)-2,6-piperidinedione; 4-[2-(3,5-dimethyl-2-oxidanyl-phenyl)-2-oxidanylidene-ethyl]piperidine-2,6-dione
|
|
| CAS | 526-02-3 | |
| PubChem CID | 245940 | |
| ChEMBL ID | CHEMBL3186963 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 275.3 | ALogp: | 1.5 |
| HBD: | 2 | HBA: | 4 |
| Rotatable Bonds: | 3 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 83.5 | Aromatic Rings: | 2 |
| Heavy Atoms: | 20 | QED Weighted: | 0.654 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.674 | MDCK Permeability: | 0.00002740 |
| Pgp-inhibitor: | 0.03 | Pgp-substrate: | 0.004 |
| Human Intestinal Absorption (HIA): | 0.004 | 20% Bioavailability (F20%): | 0.007 |
| 30% Bioavailability (F30%): | 0.01 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.45 | Plasma Protein Binding (PPB): | 74.87% |
| Volume Distribution (VD): | 0.348 | Fu: | 37.77% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.152 | CYP1A2-substrate: | 0.315 |
| CYP2C19-inhibitor: | 0.282 | CYP2C19-substrate: | 0.218 |
| CYP2C9-inhibitor: | 0.196 | CYP2C9-substrate: | 0.588 |
| CYP2D6-inhibitor: | 0.046 | CYP2D6-substrate: | 0.184 |
| CYP3A4-inhibitor: | 0.116 | CYP3A4-substrate: | 0.283 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 8.75 | Half-life (T1/2): | 0.822 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.014 | Human Hepatotoxicity (H-HT): | 0.44 |
| Drug-inuced Liver Injury (DILI): | 0.056 | AMES Toxicity: | 0.045 |
| Rat Oral Acute Toxicity: | 0.115 | Maximum Recommended Daily Dose: | 0.113 |
| Skin Sensitization: | 0.212 | Carcinogencity: | 0.052 |
| Eye Corrosion: | 0.003 | Eye Irritation: | 0.047 |
| Respiratory Toxicity: | 0.03 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC005739 | 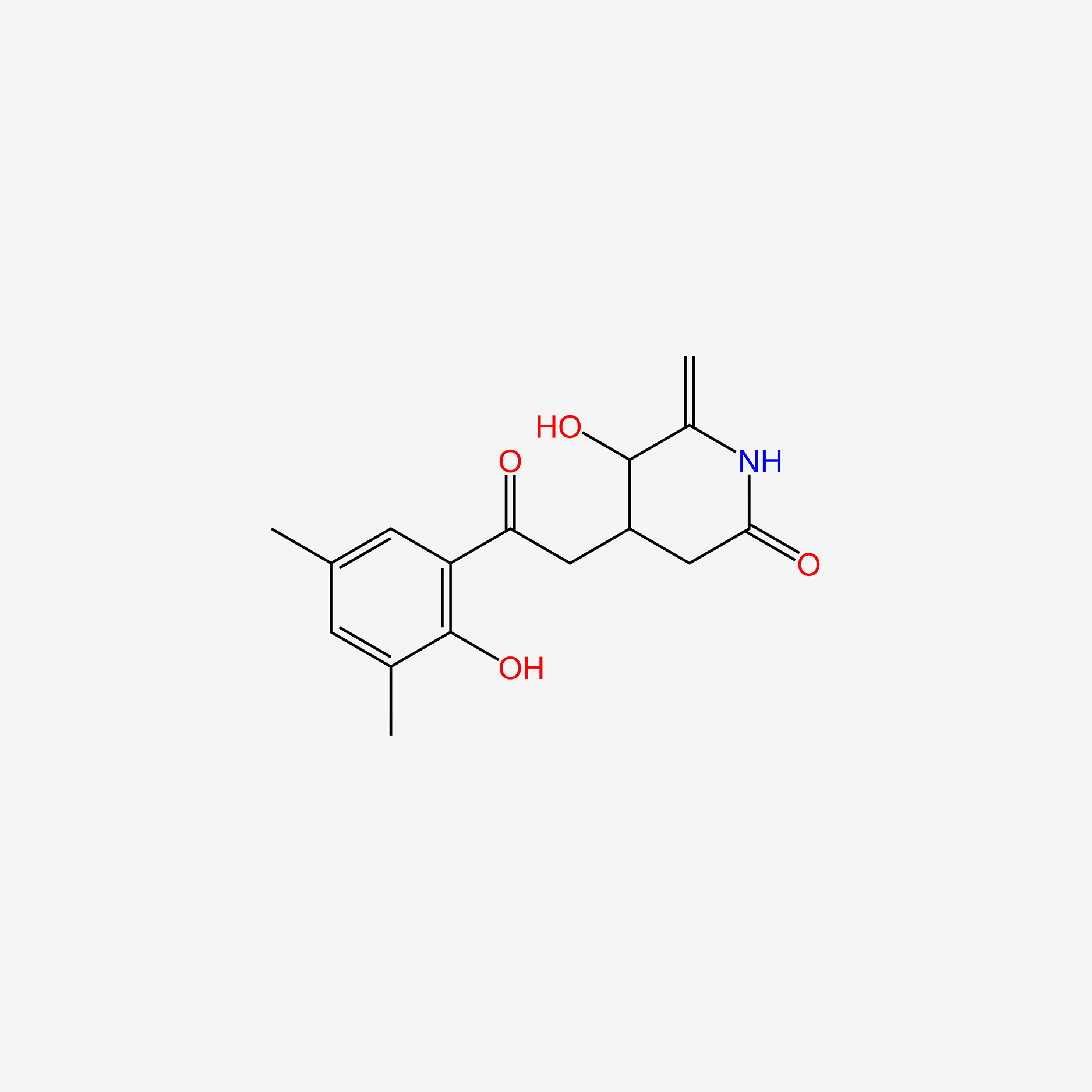 |
0.618 | D0S5CH |  |
0.253 | ||
| ENC005740 | 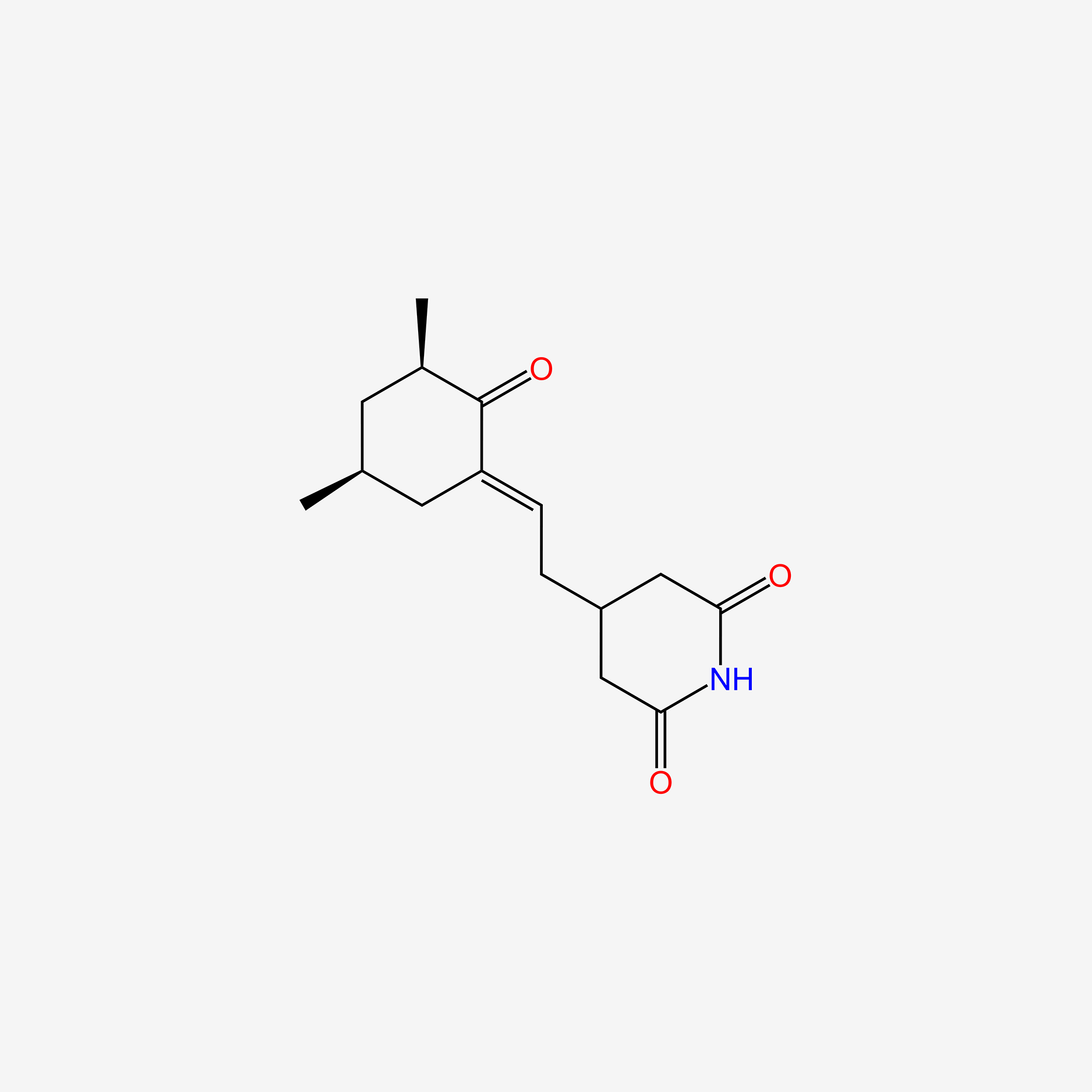 |
0.342 | D0YH0N |  |
0.250 | ||
| ENC005741 | 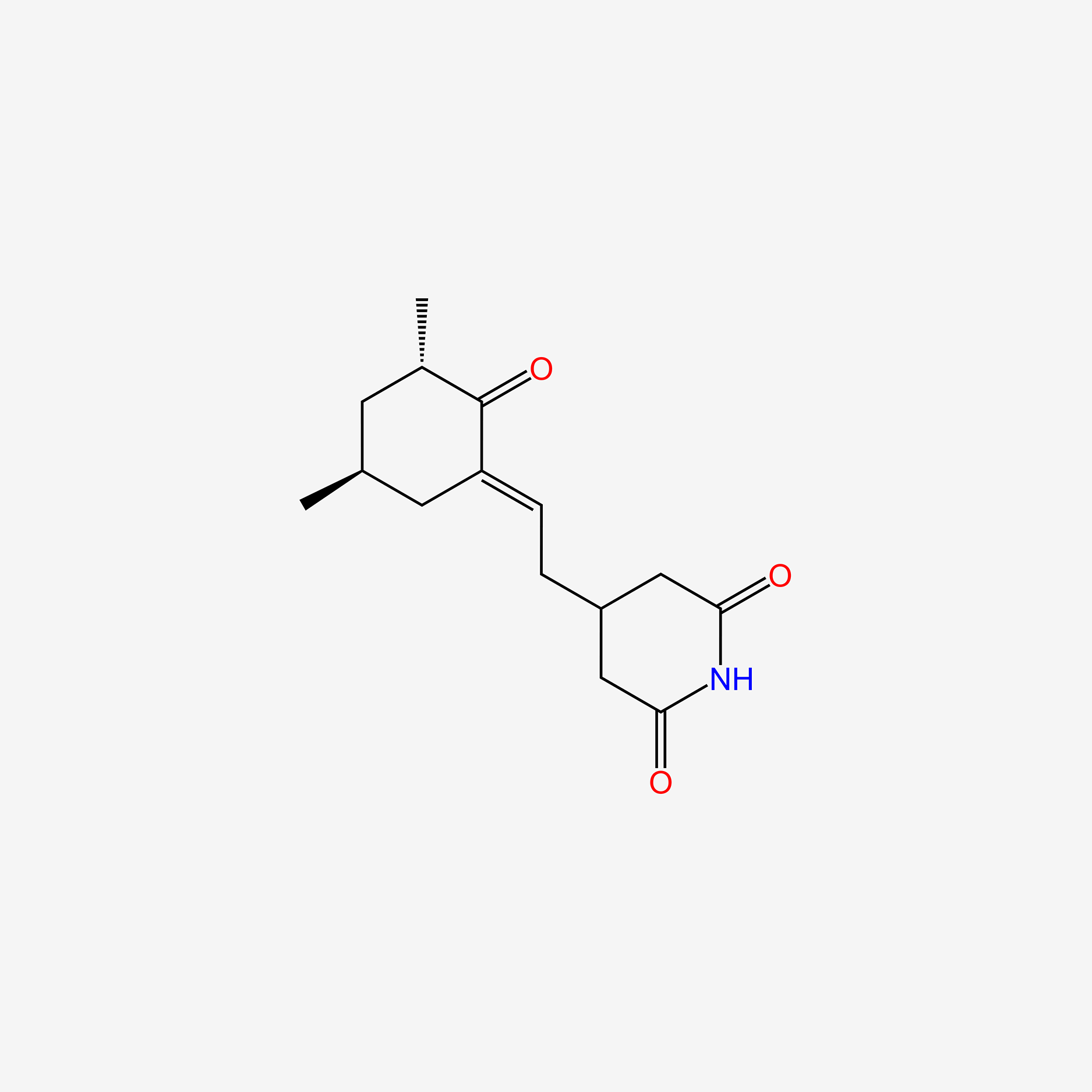 |
0.342 | D0H2ZW | 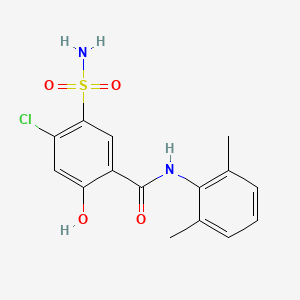 |
0.250 | ||
| ENC000131 | 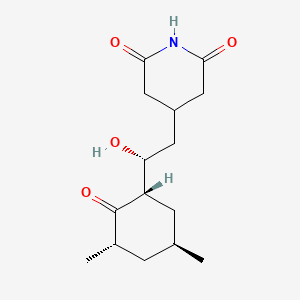 |
0.333 | D09EBS | 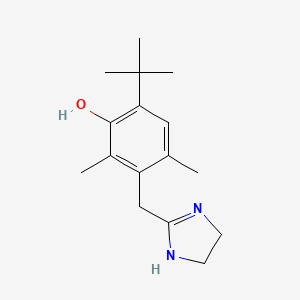 |
0.235 | ||
| ENC001890 | 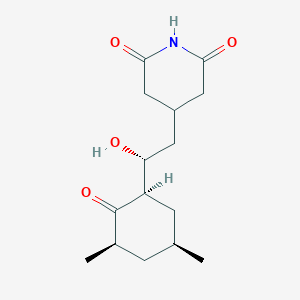 |
0.333 | D02KOF | 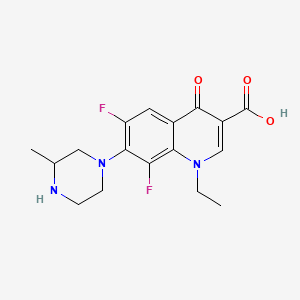 |
0.232 | ||
| ENC000869 | 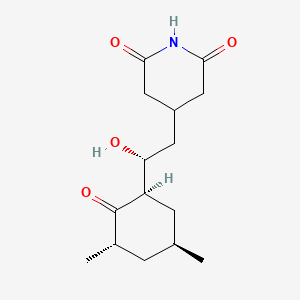 |
0.333 | D0Q2PE | 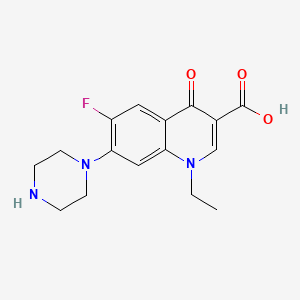 |
0.229 | ||
| ENC004789 | 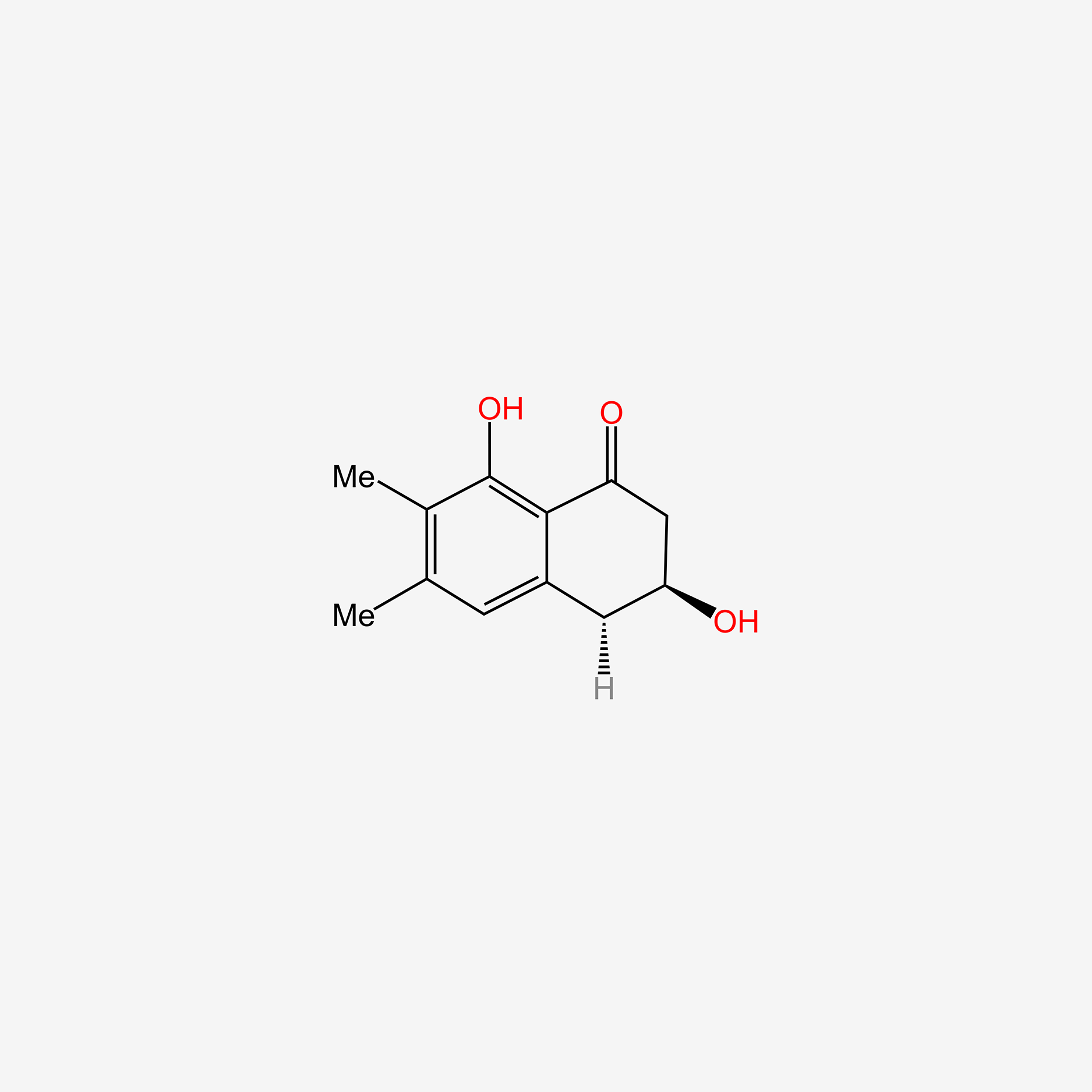 |
0.324 | D0Y7PG | 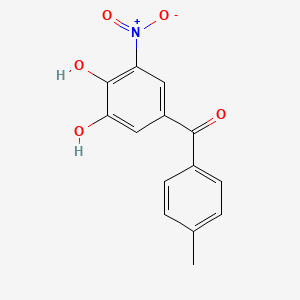 |
0.227 | ||
| ENC005530 | 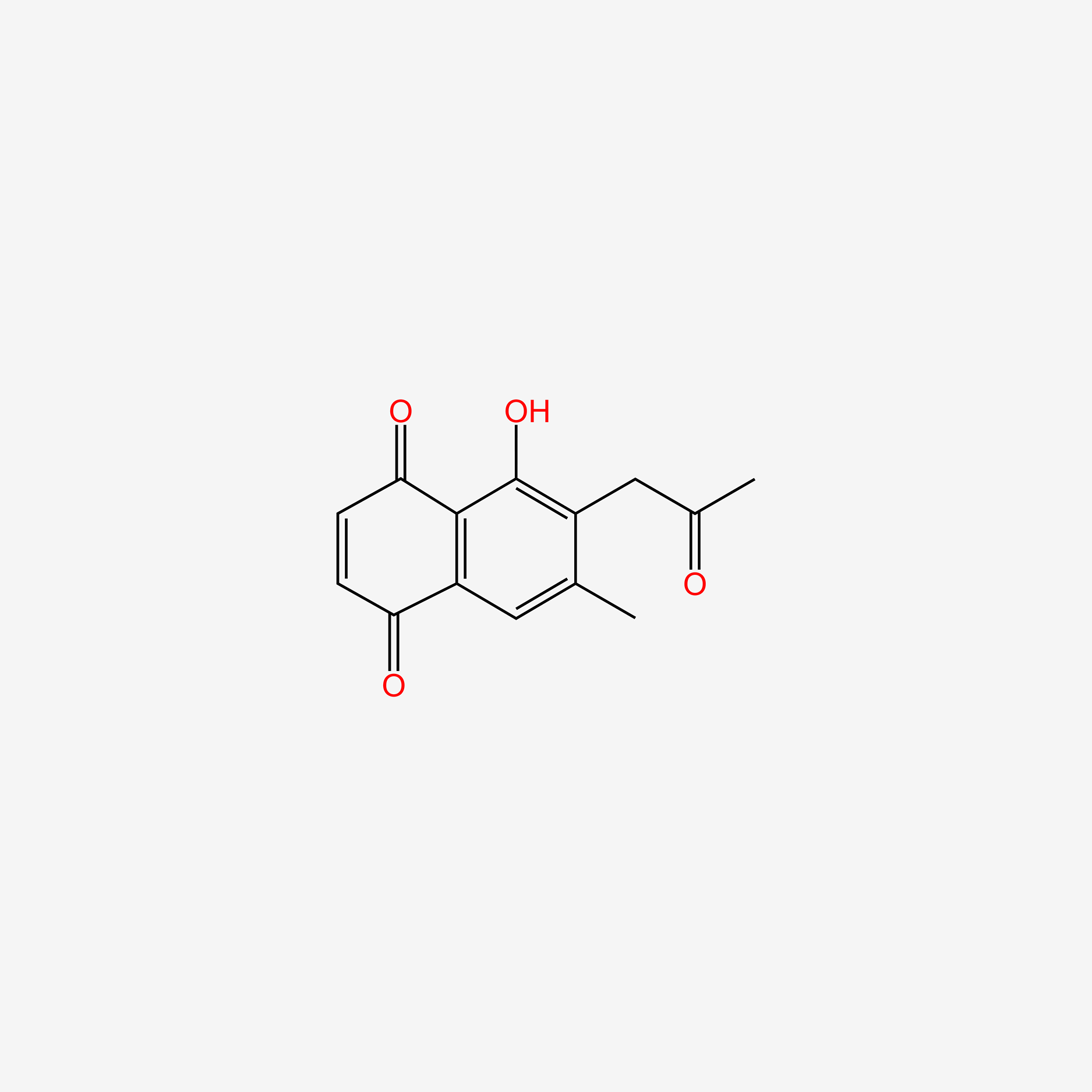 |
0.308 | D0O6KE | 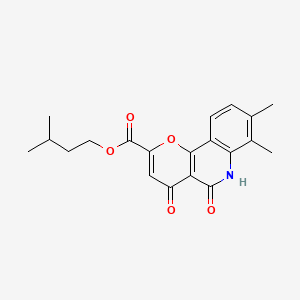 |
0.225 | ||
| ENC005639 | 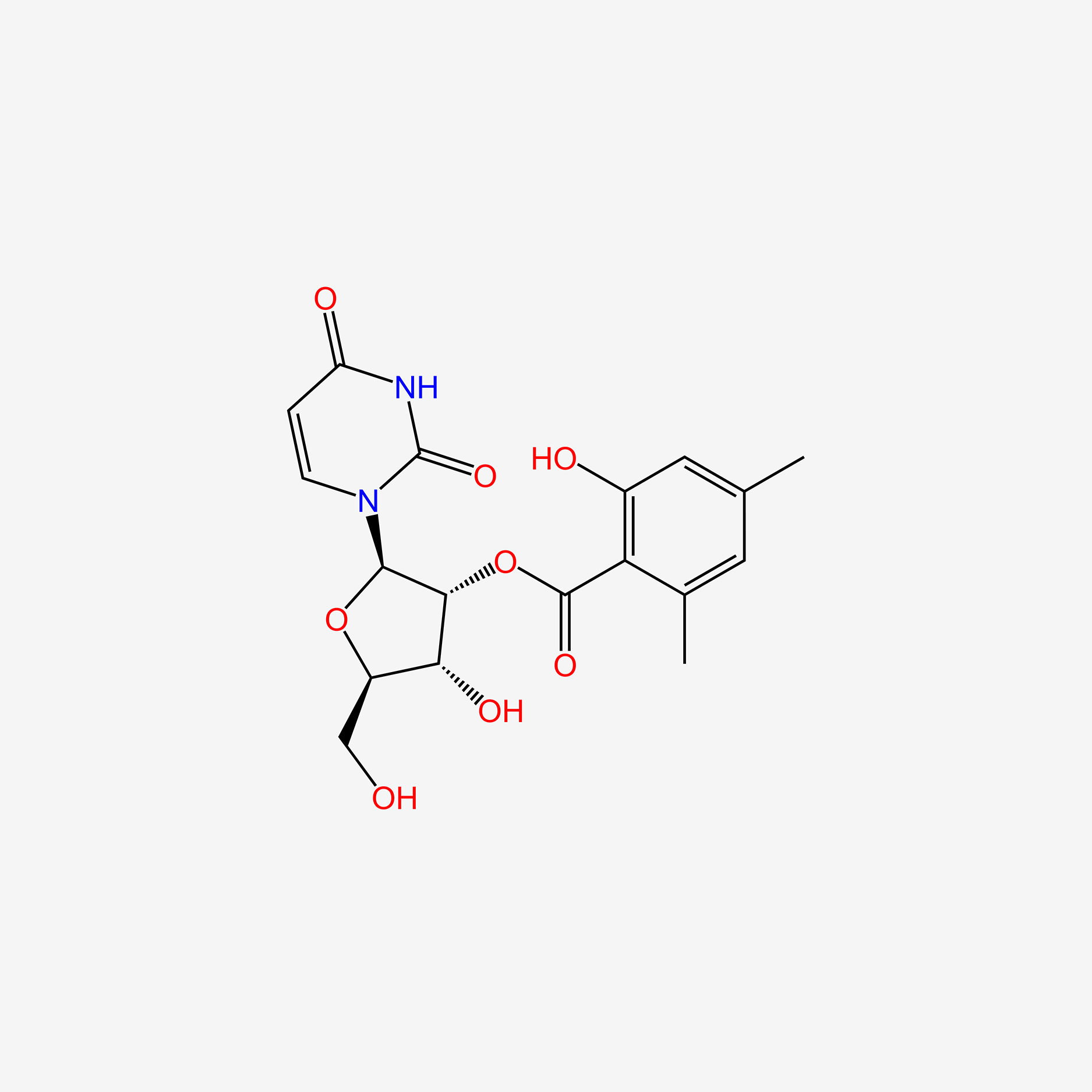 |
0.300 | D0JL2K | 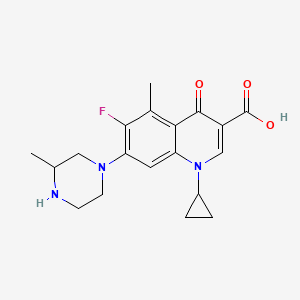 |
0.225 | ||
| ENC001498 | 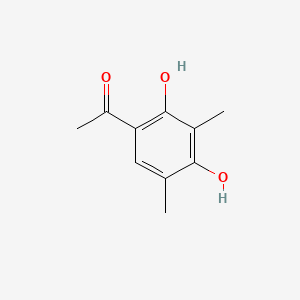 |
0.299 | D04NXQ |  |
0.224 | ||