NPs Basic Information
|
Name |
2-Hydroxy-3-methylbutyric acid
|
| Molecular Formula | C5H10O3 | |
| IUPAC Name* |
2-hydroxy-3-methylbutanoic acid
|
|
| SMILES |
CC(C)C(C(=O)O)O
|
|
| InChI |
InChI=1S/C5H10O3/c1-3(2)4(6)5(7)8/h3-4,6H,1-2H3,(H,7,8)
|
|
| InChIKey |
NGEWQZIDQIYUNV-UHFFFAOYSA-N
|
|
| Synonyms |
2-Hydroxy-3-methylbutyric acid; 2-Hydroxy-3-methylbutanoic acid; 4026-18-0; 2-Hydroxyisovaleric acid; L-Valic acid; alpha-hydroxyisovaleric acid; D-Valic acid; 2-Oxyisovaleric acid; 600-37-3; Butanoic acid, 2-hydroxy-3-methyl-; 3-Methyl-2-hydroxybutyric acid; DL-2-Hydroxyisovaleric acid; DL-2-Hydroxy-3-methylbutanoic acid; 2-Hydroxyisopentanoic acid; 02X1W97FWN; 2-Oxyisovalerate; CHEBI:60645; 2-Hydroxyisovalerate; L-alpha-Hydroxyisovaleric acid; NSC-227884; A-hydroxyisovalerate; 2-Hydroxyisopentanoate; alpha-hydroxyisovalerate; DL-a-hydroxyisovalerate; DL-2-Hydroxyisovalerate; DL-alpha-hydroxyisovalerate; 2-Hydroxy-3-methylbutyrate; 3-Methyl-2-hydroxybutyrate; DL-2-Hydroxy-3-methylbutanoate; NSC 227884; UNII-02X1W97FWN; MFCD00066442; EINECS 209-994-2; EINECS 223-697-5; (1)-2-Hydroxy-3-methylbutyric acid; NSC227884; (+/-)-2-Hydroxy-3-methylbutyric acid; A-hydroxyisovaleric acid; DL-a-hydroxyisovaleric acid; SCHEMBL43434; (RS)-2-hydroxyisovaleric acid; 2-Hydroxy-3-methylbutanoicacid; DL-alpha-hydroxyisovaleric acid; ( inverted exclamation markA)-2-Hydroxyisopentanoic Acid; CHEMBL1162479; NGEWQZIDQIYUNV-UHFFFAOYSA-; 2-Hydroxy-3-methyl butyric acid; DTXSID10863305; 3-methyl-2-oxidanyl-butanoic acid; 2-Hydroxy-3-methylbutanoic acid #; AMY22056; BCP33333; BCP33335; D-ALPHA-HYDROXYISOVALERICACID; LMFA01050478; MFCD00004242; s6098; .ALPHA.-HYDROXYISOVALERIC ACID; AKOS000278106; AKOS016182980; 2-Hydroxy-3-methylbutyric acid, 99%; AB88448; CS-W008150; HY-W008150; (+/-)-2-HYDROXYISOVALERIC ACID; DL-.ALPHA.-HYDROXYISOVALERIC ACID; AS-12349; SY041974; (+/-)-2-HYDROXYISOPENTANOIC ACID; Butanoic acid,2-hydroxy-3-methyl-,(2R)-; BUTYRIC ACID, 2-HYDROXY-3-METHYL-; DB-021168; FT-0635106; FT-0659220; FT-0691710; (+/-)-.ALPHA.-HYDROXYISOVALERIC ACID; 2-Methylpyridine-4-boronic?acid?pinacol?ester; EN300-115018; F19720; 026H180; A825017; W-106368; Q27104874; (R)-2-Hydroxyisovaleric acid;D-alpha-Hydroxyisovaleric acid
|
|
| CAS | 4026-18-0 | |
| PubChem CID | 99823 | |
| ChEMBL ID | CHEMBL1162479 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 118.13 | ALogp: | 0.5 |
| HBD: | 2 | HBA: | 3 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 57.5 | Aromatic Rings: | 0 |
| Heavy Atoms: | 8 | QED Weighted: | 0.554 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.635 | MDCK Permeability: | 0.00185664 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.268 |
| Human Intestinal Absorption (HIA): | 0.009 | 20% Bioavailability (F20%): | 0.035 |
| 30% Bioavailability (F30%): | 0.02 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.522 | Plasma Protein Binding (PPB): | 27.42% |
| Volume Distribution (VD): | 1.102 | Fu: | 74.93% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.02 | CYP1A2-substrate: | 0.413 |
| CYP2C19-inhibitor: | 0.02 | CYP2C19-substrate: | 0.819 |
| CYP2C9-inhibitor: | 0.005 | CYP2C9-substrate: | 0.113 |
| CYP2D6-inhibitor: | 0.006 | CYP2D6-substrate: | 0.212 |
| CYP3A4-inhibitor: | 0.005 | CYP3A4-substrate: | 0.138 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 3.383 | Half-life (T1/2): | 0.875 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.011 | Human Hepatotoxicity (H-HT): | 0.193 |
| Drug-inuced Liver Injury (DILI): | 0.804 | AMES Toxicity: | 0.31 |
| Rat Oral Acute Toxicity: | 0.054 | Maximum Recommended Daily Dose: | 0.162 |
| Skin Sensitization: | 0.49 | Carcinogencity: | 0.225 |
| Eye Corrosion: | 0.028 | Eye Irritation: | 0.985 |
| Respiratory Toxicity: | 0.396 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
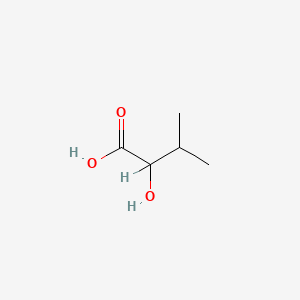
| ENC000149 | 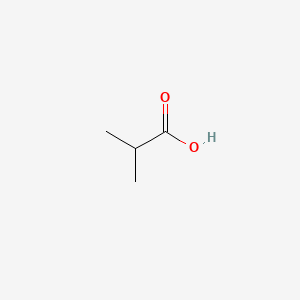 |
0.524 | D08QGD | 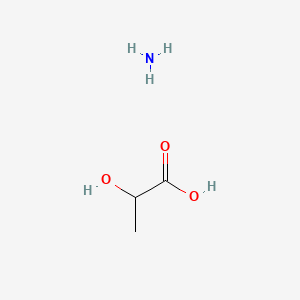 |
0.500 | ||
| ENC000037 | 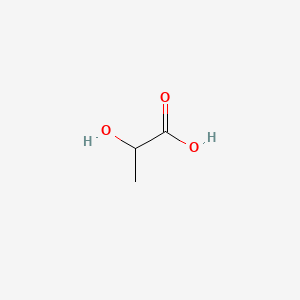 |
0.524 | D09PUL | 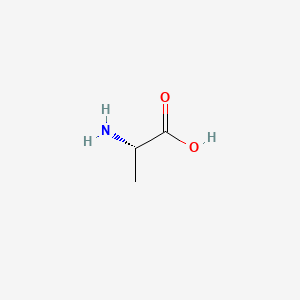 |
0.391 | ||
| ENC004134 |  |
0.444 | D00ZOF | 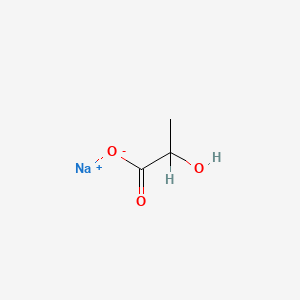 |
0.320 | ||
| ENC001011 | 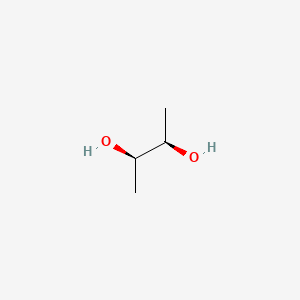 |
0.391 | D00WUF | 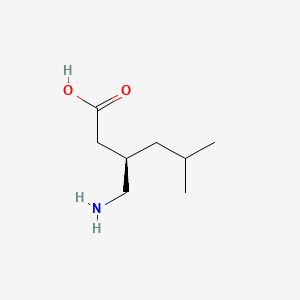 |
0.306 | ||
| ENC000010 | 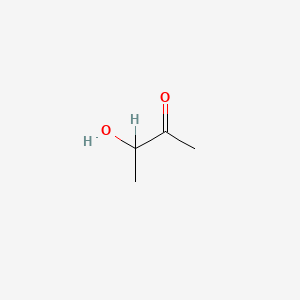 |
0.391 | D08HZC | 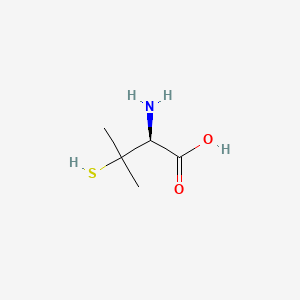 |
0.290 | ||
| ENC000016 | 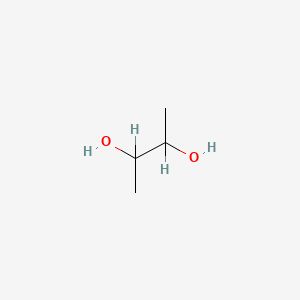 |
0.391 | D02UDJ | 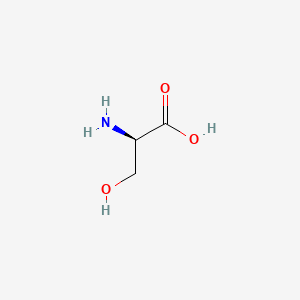 |
0.286 | ||
| ENC000031 | 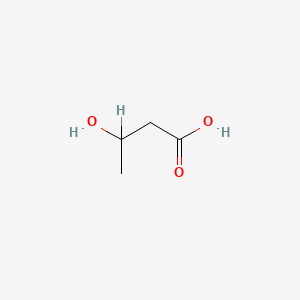 |
0.385 | D04CRL | 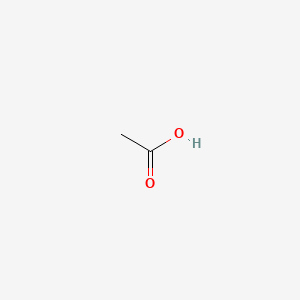 |
0.286 | ||
| ENC000289 | 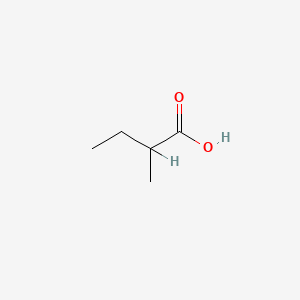 |
0.385 | D01GYK | 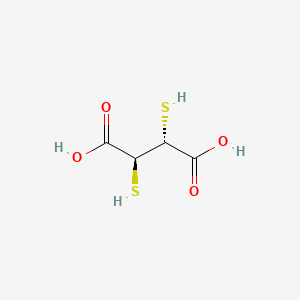 |
0.265 | ||
| ENC000351 | 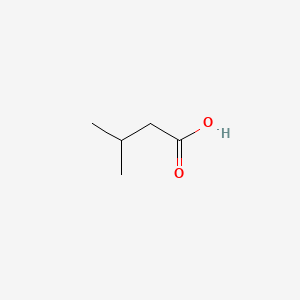 |
0.385 | D0R1QE |  |
0.255 | ||
| ENC000890 | 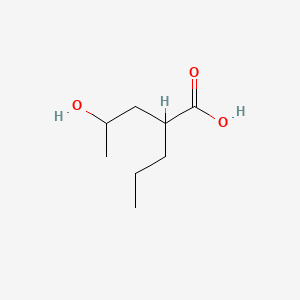 |
0.382 | D0Y3KG |  |
0.250 | ||