NPs Basic Information

|
Name |
2-Butylfuran
|
| Molecular Formula | C8H12O | |
| IUPAC Name* |
2-butylfuran
|
|
| SMILES |
CCCCC1=CC=CO1
|
|
| InChI |
InChI=1S/C8H12O/c1-2-3-5-8-6-4-7-9-8/h4,6-7H,2-3,5H2,1H3
|
|
| InChIKey |
NWZIYQNUCXUJJJ-UHFFFAOYSA-N
|
|
| Synonyms |
2-BUTYLFURAN; 4466-24-4; Furan, 2-butyl-; 2-n-Butylfuran; 2-n-Butyl furan; 2-butyl furan; 81JV9ZYK0D; 2-butyl-furan; UNII-81JV9ZYK0D; EINECS 224-732-7; 2-(But-1-yl)fura; 2-(But-1-yl)furan; 2-Butylfuran, AldrichCPR; 2-BUTYLFURAN [FHFI]; SCHEMBL256700; DTXSID8073340; FEMA NO. 4081; CHEBI:89750; NWZIYQNUCXUJJJ-UHFFFAOYSA-; ZINC2037803; MFCD00047071; AKOS025396869; PS-4850; SB60946; 2-Butylfuran 100 microg/mL in Acetonitrile; B2412; CS-0152344; FT-0613148; 2-Butylfuran 1000 microg/mL in Acetonitrile; D88935; EN300-7396471; Q27161938
|
|
| CAS | 4466-24-4 | |
| PubChem CID | 20534 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 124.18 | ALogp: | 3.1 |
| HBD: | 0 | HBA: | 1 |
| Rotatable Bonds: | 3 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 13.1 | Aromatic Rings: | 1 |
| Heavy Atoms: | 9 | QED Weighted: | 0.601 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.232 | MDCK Permeability: | 0.00002560 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.278 |
| Human Intestinal Absorption (HIA): | 0.003 | 20% Bioavailability (F20%): | 0.555 |
| 30% Bioavailability (F30%): | 0.298 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.627 | Plasma Protein Binding (PPB): | 94.27% |
| Volume Distribution (VD): | 2.453 | Fu: | 6.92% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.981 | CYP1A2-substrate: | 0.89 |
| CYP2C19-inhibitor: | 0.775 | CYP2C19-substrate: | 0.388 |
| CYP2C9-inhibitor: | 0.535 | CYP2C9-substrate: | 0.666 |
| CYP2D6-inhibitor: | 0.03 | CYP2D6-substrate: | 0.424 |
| CYP3A4-inhibitor: | 0.029 | CYP3A4-substrate: | 0.285 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 9.978 | Half-life (T1/2): | 0.701 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.075 | Human Hepatotoxicity (H-HT): | 0.081 |
| Drug-inuced Liver Injury (DILI): | 0.164 | AMES Toxicity: | 0.033 |
| Rat Oral Acute Toxicity: | 0.715 | Maximum Recommended Daily Dose: | 0.037 |
| Skin Sensitization: | 0.296 | Carcinogencity: | 0.59 |
| Eye Corrosion: | 0.968 | Eye Irritation: | 0.993 |
| Respiratory Toxicity: | 0.907 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000533 |  |
0.833 | D03OIW |  |
0.254 | ||
| ENC000534 |  |
0.694 | D0L7UQ |  |
0.229 | ||
| ENC000189 | 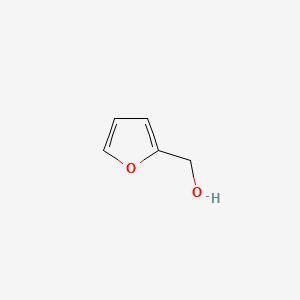 |
0.484 | D02HXS |  |
0.224 | ||
| ENC000617 |  |
0.463 | D0PQ3G | 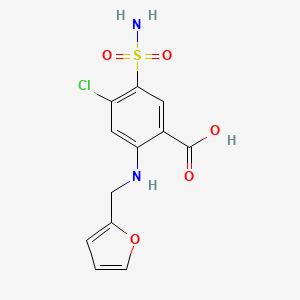 |
0.221 | ||
| ENC000480 |  |
0.333 | D0P2GK |  |
0.220 | ||
| ENC000096 |  |
0.319 | D06OIV |  |
0.210 | ||
| ENC000190 |  |
0.314 | D01QLH |  |
0.200 | ||
| ENC001133 |  |
0.308 | D0A0FL |  |
0.197 | ||
| ENC000217 |  |
0.300 | D09QUQ |  |
0.197 | ||
| ENC000162 | 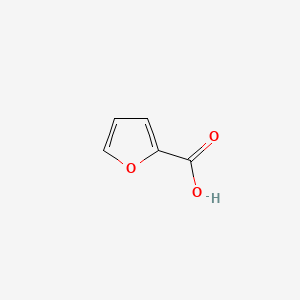 |
0.297 | D08HQK |  |
0.197 | ||