NPs Basic Information

|
Name |
2-Pentylfuran
|
| Molecular Formula | C9H14O | |
| IUPAC Name* |
2-pentylfuran
|
|
| SMILES |
CCCCCC1=CC=CO1
|
|
| InChI |
InChI=1S/C9H14O/c1-2-3-4-6-9-7-5-8-10-9/h5,7-8H,2-4,6H2,1H3
|
|
| InChIKey |
YVBAUDVGOFCUSG-UHFFFAOYSA-N
|
|
| Synonyms |
2-PENTYLFURAN; 3777-69-3; 2-Amylfuran; 2-n-Pentylfuran; Furan, 2-pentyl-; Furan, pentyl-; PENTYLFURAN; FEMA No. 3317; 2-pentyl furan; 6I0QAJ1JZQ; CHEBI:89197; 2-Pentylfuran (natural); 2-(N-Pentyl)furan; EINECS 223-234-7; UNII-6I0QAJ1JZQ; BRN 0107854; 2-pentylfurane; CCRIS 8807; 2-pentyl-furan; Furane, 2-pentyl; MFCD00036497; 2-(Pent-1-yl)fura; DSSTox_CID_27679; DSSTox_RID_82496; DSSTox_GSID_47679; 5-17-01-00390 (Beilstein Handbook Reference); 2-PENTYLFURAN [FHFI]; Amyl furan (2-Pentyl furan); SCHEMBL221257; CHEMBL3182720; DTXSID9047679; 2-Pentylfuran, >=98%, FG; FEMA 3317; 2-Pentylfuran, analytical standard; HY-N7398; ZINC1997926; Tox21_303542; s9334; AKOS015913798; SB61015; NCGC00257337-01; 64079-01-2; BS-22948; CAS-3777-69-3; DB-003325; CS-0119428; FT-0613265; P1209; 2-Pentylfuran 100 microg/mL in Acetonitrile; 2-Pentylfuran, natural (US), >=97%, FG; H11330; EN300-7399562; 777P693; A823863; W-106514; Q27161382
|
|
| CAS | 3777-69-3 | |
| PubChem CID | 19602 | |
| ChEMBL ID | CHEMBL3182720 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 138.21 | ALogp: | 3.7 |
| HBD: | 0 | HBA: | 1 |
| Rotatable Bonds: | 4 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 13.1 | Aromatic Rings: | 1 |
| Heavy Atoms: | 10 | QED Weighted: | 0.577 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.272 | MDCK Permeability: | 0.00002120 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.138 |
| Human Intestinal Absorption (HIA): | 0.003 | 20% Bioavailability (F20%): | 0.642 |
| 30% Bioavailability (F30%): | 0.853 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.611 | Plasma Protein Binding (PPB): | 95.48% |
| Volume Distribution (VD): | 2.485 | Fu: | 4.47% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.983 | CYP1A2-substrate: | 0.853 |
| CYP2C19-inhibitor: | 0.817 | CYP2C19-substrate: | 0.245 |
| CYP2C9-inhibitor: | 0.662 | CYP2C9-substrate: | 0.7 |
| CYP2D6-inhibitor: | 0.038 | CYP2D6-substrate: | 0.34 |
| CYP3A4-inhibitor: | 0.046 | CYP3A4-substrate: | 0.259 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 9.353 | Half-life (T1/2): | 0.616 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.089 | Human Hepatotoxicity (H-HT): | 0.077 |
| Drug-inuced Liver Injury (DILI): | 0.184 | AMES Toxicity: | 0.031 |
| Rat Oral Acute Toxicity: | 0.611 | Maximum Recommended Daily Dose: | 0.036 |
| Skin Sensitization: | 0.409 | Carcinogencity: | 0.508 |
| Eye Corrosion: | 0.97 | Eye Irritation: | 0.993 |
| Respiratory Toxicity: | 0.911 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000546 |  |
0.833 | D01QLH |  |
0.275 | ||
| ENC000534 |  |
0.829 | D07UHS |  |
0.247 | ||
| ENC000617 |  |
0.575 | D03OIW |  |
0.242 | ||
| ENC000189 | 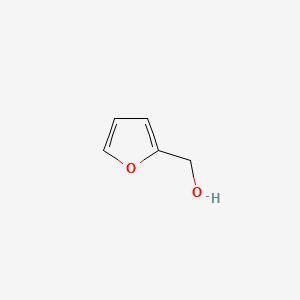 |
0.441 | D0P1FO | 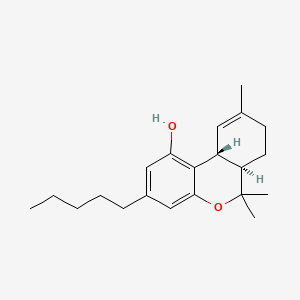 |
0.240 | ||
| ENC004625 |  |
0.367 | D02HXS |  |
0.233 | ||
| ENC000861 |  |
0.333 | D0O1UZ | 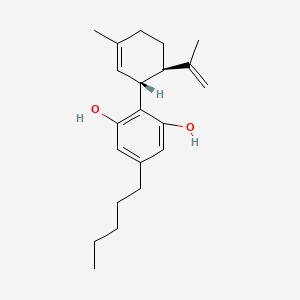 |
0.227 | ||
| ENC000139 | 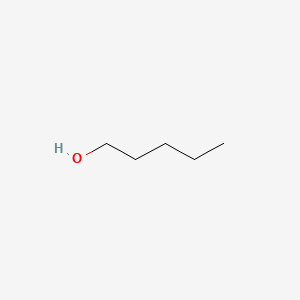 |
0.324 | D0L7UQ |  |
0.216 | ||
| ENC000480 |  |
0.308 | D0V4UF | 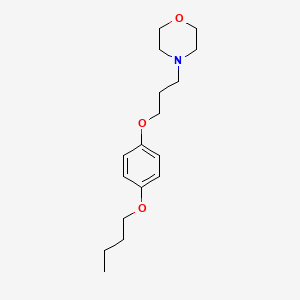 |
0.213 | ||
| ENC004381 | 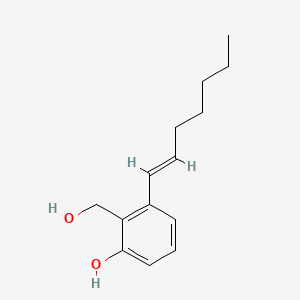 |
0.298 | D0PQ3G | 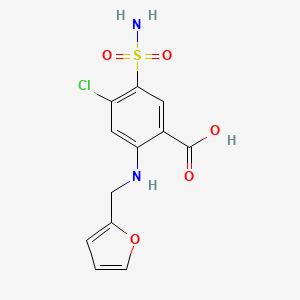 |
0.211 | ||
| ENC001477 | 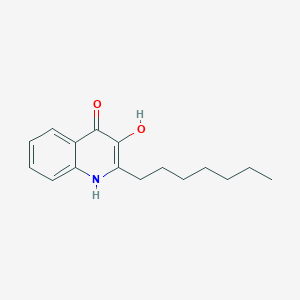 |
0.297 | D0O2YE |  |
0.211 | ||