NPs Basic Information
|
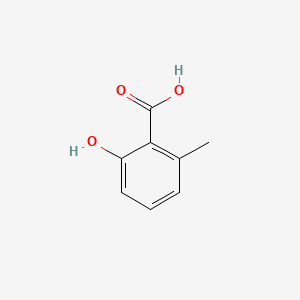
Name |
2-Hydroxy-6-methylbenzoic acid
|
| Molecular Formula | C8H8O3 | |
| IUPAC Name* |
2-hydroxy-6-methylbenzoic acid
|
|
| SMILES |
CC1=C(C(=CC=C1)O)C(=O)O
|
|
| InChI |
InChI=1S/C8H8O3/c1-5-3-2-4-6(9)7(5)8(10)11/h2-4,9H,1H3,(H,10,11)
|
|
| InChIKey |
HCJMNOSIAGSZBM-UHFFFAOYSA-N
|
|
| Synonyms |
2-HYDROXY-6-METHYLBENZOIC ACID; 567-61-3; 6-Methylsalicylic acid; 2,6-Cresotic acid; 6-Hydroxy-o-toluic acid; Benzoic acid, 2-hydroxy-6-methyl-; 6-MSA; 6-MS; CHEBI:17637; L5352FE23Y; 6-Methyl-2-hydroxybenzenecarboxylate; MFCD01194284; NSC-403256; Methylsalicylic acid; NSC 403256; BRN 2208693; 2-Hydroxy-6-methylbenzoicacid; UNII-L5352FE23Y; Methylsalicylic acid, 6-; 4-10-00-00594 (Beilstein Handbook Reference); SCHEMBL147955; CHEMBL510026; DTXSID20205257; 2-Hydroxy-6-methylbenzoic acid #; ZINC901434; ACT12250; BCP05389; AM1081; GEO-04151; LMPK13010002; NSC403256; AKOS004907081; AC-5291; CS-W002751; FS-2603; MB01720; SY030546; DB-006156; A8114; FT-0600234; C02657; EN300-105059; 567H613; J-509658; J-512620; Q6172528
|
|
| CAS | 567-61-3 | |
| PubChem CID | 11279 | |
| ChEMBL ID | CHEMBL510026 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 152.15 | ALogp: | 1.9 |
| HBD: | 2 | HBA: | 3 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 57.5 | Aromatic Rings: | 1 |
| Heavy Atoms: | 11 | QED Weighted: | 0.646 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.174 | MDCK Permeability: | 0.00001020 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.005 |
| Human Intestinal Absorption (HIA): | 0.009 | 20% Bioavailability (F20%): | 0.008 |
| 30% Bioavailability (F30%): | 0.197 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.235 | Plasma Protein Binding (PPB): | 80.47% |
| Volume Distribution (VD): | 0.345 | Fu: | 16.60% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.114 | CYP1A2-substrate: | 0.239 |
| CYP2C19-inhibitor: | 0.05 | CYP2C19-substrate: | 0.056 |
| CYP2C9-inhibitor: | 0.105 | CYP2C9-substrate: | 0.159 |
| CYP2D6-inhibitor: | 0.04 | CYP2D6-substrate: | 0.139 |
| CYP3A4-inhibitor: | 0.04 | CYP3A4-substrate: | 0.093 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 3.92 | Half-life (T1/2): | 0.886 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.045 | Human Hepatotoxicity (H-HT): | 0.521 |
| Drug-inuced Liver Injury (DILI): | 0.901 | AMES Toxicity: | 0.024 |
| Rat Oral Acute Toxicity: | 0.501 | Maximum Recommended Daily Dose: | 0.008 |
| Skin Sensitization: | 0.375 | Carcinogencity: | 0.099 |
| Eye Corrosion: | 0.045 | Eye Irritation: | 0.99 |
| Respiratory Toxicity: | 0.692 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000690 |  |
0.568 | D07HBX |  |
0.474 | ||
| ENC000674 |  |
0.500 | D0C4YC |  |
0.415 | ||
| ENC001513 |  |
0.488 | D01WJL | 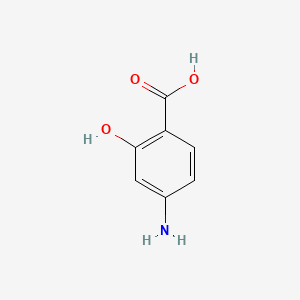 |
0.415 | ||
| ENC000404 |  |
0.472 | D01PJR |  |
0.347 | ||
| ENC002350 |  |
0.467 | D0F5ZM | 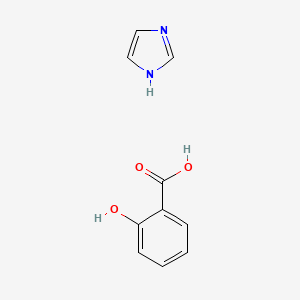 |
0.340 | ||
| ENC000028 |  |
0.457 | D05FTJ | 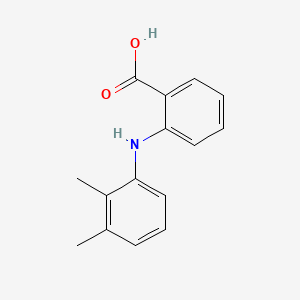 |
0.339 | ||
| ENC002237 |  |
0.457 | D0BA6T | 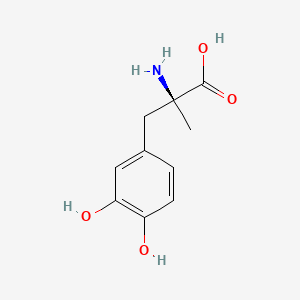 |
0.333 | ||
| ENC006051 |  |
0.441 | D0V9EN | 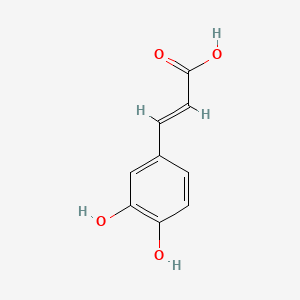 |
0.333 | ||
| ENC004796 |  |
0.438 | D08HVR | 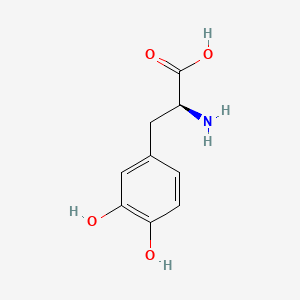 |
0.320 | ||
| ENC000296 |  |
0.419 | D0P7JZ | 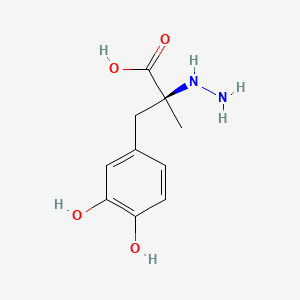 |
0.315 | ||