NPs Basic Information
|
Name |
M-Cymene
|
| Molecular Formula | C10H14 | |
| IUPAC Name* |
1-methyl-3-propan-2-ylbenzene
|
|
| SMILES |
CC1=CC(=CC=C1)C(C)C
|
|
| InChI |
InChI=1S/C10H14/c1-8(2)10-6-4-5-9(3)7-10/h4-8H,1-3H3
|
|
| InChIKey |
XCYJPXQACVEIOS-UHFFFAOYSA-N
|
|
| Synonyms |
M-CYMENE; 535-77-3; 1-Isopropyl-3-methylbenzene; 3-Isopropyltoluene; m-Isopropyltoluene; m-Cymol; m-Methylisopropylbenzene; 1-Methyl-3-isopropylbenzene; beta-Cymene; Benzene, 1-methyl-3-(1-methylethyl)-; 1-Methyl-3-(1-methylethyl)benzene; 1-methyl-3-propan-2-ylbenzene; meta-cymene; .beta.-Cymene; 3-Methyl-1-isopropylbenzene; NSC 73975; 10ZH8R921S; 1-Methyl-3-(1-methylethyl)-benzene; NSC-73975; HSDB 3428; EINECS 208-617-9; BRN 1851357; UNII-10ZH8R921S; 1-methyl-3-(propan-2-yl)benzene; m-cymene [UN2046] [Flammable liquid]; m-Cymene, 99%; 3-Methylisopropylbenzene; m-cymene [UN2046] [Flammable liquid]; M-CYMENE [HSDB]; CYMENE, M-; M-CYMENE [MI]; m-Mentha-1,3,5-triene; 4-05-00-01058 (Beilstein Handbook Reference); m-Cymene, analytical standard; 1-Methyl-3-isopropyl benzene; DTXSID2060206; NSC73975; ZINC1699438; MFCD00008891; 1-methyl-3-(1-methylethyl) benzene; AKOS005110997; 1-Methyl-3-(1-methylethyl)benzene, 9CI; C0798; CS-0331846; FT-0682671; T71023; Q27251197
|
|
| CAS | 535-77-3 | |
| PubChem CID | 10812 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 134.22 | ALogp: | 4.0 |
| HBD: | 0 | HBA: | 0 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 0.0 | Aromatic Rings: | 1 |
| Heavy Atoms: | 10 | QED Weighted: | 0.546 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.31 | MDCK Permeability: | 0.00001960 |
| Pgp-inhibitor: | 0.008 | Pgp-substrate: | 0.006 |
| Human Intestinal Absorption (HIA): | 0.004 | 20% Bioavailability (F20%): | 0.171 |
| 30% Bioavailability (F30%): | 0.943 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.842 | Plasma Protein Binding (PPB): | 94.10% |
| Volume Distribution (VD): | 2.531 | Fu: | 6.61% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.936 | CYP1A2-substrate: | 0.934 |
| CYP2C19-inhibitor: | 0.82 | CYP2C19-substrate: | 0.862 |
| CYP2C9-inhibitor: | 0.499 | CYP2C9-substrate: | 0.459 |
| CYP2D6-inhibitor: | 0.768 | CYP2D6-substrate: | 0.644 |
| CYP3A4-inhibitor: | 0.129 | CYP3A4-substrate: | 0.48 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.461 | Half-life (T1/2): | 0.392 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.026 | Human Hepatotoxicity (H-HT): | 0.051 |
| Drug-inuced Liver Injury (DILI): | 0.145 | AMES Toxicity: | 0.015 |
| Rat Oral Acute Toxicity: | 0.083 | Maximum Recommended Daily Dose: | 0.064 |
| Skin Sensitization: | 0.149 | Carcinogencity: | 0.215 |
| Eye Corrosion: | 0.963 | Eye Irritation: | 0.994 |
| Respiratory Toxicity: | 0.034 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
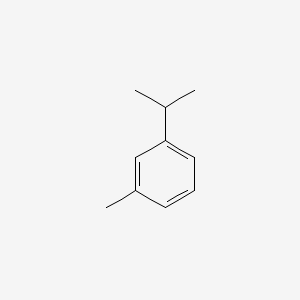
| ENC000199 | 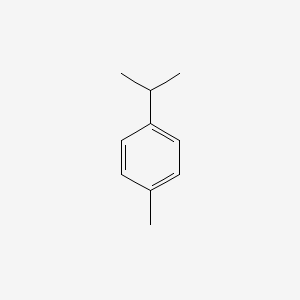 |
0.543 | D06GIP |  |
0.474 | ||
| ENC000239 |  |
0.531 | D0EL2O |  |
0.386 | ||
| ENC000347 |  |
0.514 | D04EYC | 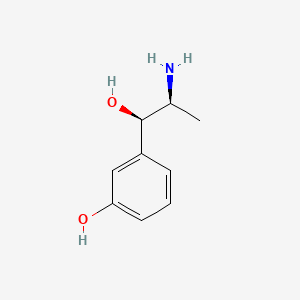 |
0.372 | ||
| ENC000191 |  |
0.486 | D0A3HB |  |
0.356 | ||
| ENC000413 |  |
0.486 | D0K4MH |  |
0.346 | ||
| ENC000365 |  |
0.459 | D0O6IU | 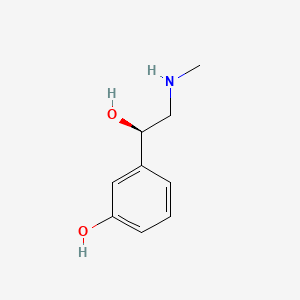 |
0.333 | ||
| ENC000414 |  |
0.444 | D0X0RI |  |
0.319 | ||
| ENC000471 | 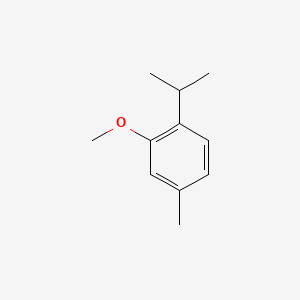 |
0.439 | D0WY5Q | 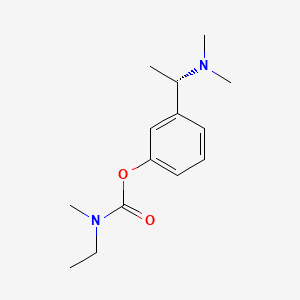 |
0.316 | ||
| ENC000370 |  |
0.425 | D0S5LH |  |
0.311 | ||
| ENC000098 |  |
0.417 | D01PJR |  |
0.306 | ||