NPs Basic Information

|
Name |
Elemicin
|
| Molecular Formula | C12H16O3 | |
| IUPAC Name* |
1,2,3-trimethoxy-5-prop-2-enylbenzene
|
|
| SMILES |
COC1=CC(=CC(=C1OC)OC)CC=C
|
|
| InChI |
InChI=1S/C12H16O3/c1-5-6-9-7-10(13-2)12(15-4)11(8-9)14-3/h5,7-8H,1,6H2,2-4H3
|
|
| InChIKey |
BPLQKQKXWHCZSS-UHFFFAOYSA-N
|
|
| Synonyms |
Elemicin; 487-11-6; 5-Allyl-1,2,3-trimethoxybenzene; Elemicine; 3,4,5-Trimethoxyallylbenzene; 1,2,3-trimethoxy-5-prop-2-enylbenzene; Benzene, 1,2,3-trimethoxy-5-(2-propenyl)-; 1,2,3-Trimethoxy-5-(2-propenyl)benzene; BENZENE, 5-ALLYL-1,2,3-TRIMETHOXY-; 3-(3,4,5-Trimethoxyphenyl)-1-propene; 4-allyl-1,2,6-trimethoxybenzene; HSZ191AKAN; 3,4,5-trimethoxyallyl benzene; CHEBI:4771; 1,2,3-trimethoxy-5-(prop-2-en-1-yl)benzene; MFCD01656688; NSC-16704; 1,2,3-Trimethoxy-5-(2-propenyl)-benzene; Benzene, 5-(2-propenyl)-1,2,3-trimethoxy; 1,2,3-Trimethoxy-5-[2-propenyl]-benzene; CCRIS 6783; EINECS 207-649-0; UNII-HSZ191AKAN; NSC 16704; BRN 1912664; AI3-20815; 5'-metoxy eugenol; 5-allyl-1,2,3-trimethoxy-benzene; 3,5-Trimethoxyallylbenzene; 5-(Prop-2-en-1-yl)-1,2,3-trimethoxybenzene; SCHEMBL68542; 4-06-00-07478 (Beilstein Handbook Reference); 3,4, 5-Trimethoxyallylbenzene; CHEMBL458690; DTXSID60197586; 1,2,3-Trimethoxy-5-allylbenzene; ZINC899845; HY-N6807; NSC16704; s5120; AKOS015896443; CCG-266643; PS-4970; 1-ALLYL-3,4,5-TRIMETHOXYBENZENE; 1,2,3-trimethoxy-5-prop-2-enyl-benzene; Benzene,2,3-trimethoxy-5-(2-propenyl)-; DB-081347; 1,2,3-Trimethoxy-5-allylbenzene (elemicin); CS-0030665; FT-0652063; 1,2,3-Trimethoxy-5-(2-propenyl)benzene, 9CI; 1-(3,4,5-TRIMETHOXYPHENYL)-2-PROPENE; 487A116; A827594; Q417746; 4-(2-Ethyl-benzoimidazol-1-yl)-4-oxo-butyricacid; 5-(PROP-2-ENYL)-1,2,3-TRIMETHOXYBENZENE; J-520432; Benzene, 1,2,3-trimethoxy-5-(2-propenyl)- (9CI); BENZENE, 1,2,3-TRIMETHOXY-5-(2-PROPEN-1-YL)-
|
|
| CAS | 487-11-6 | |
| PubChem CID | 10248 | |
| ChEMBL ID | CHEMBL458690 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 208.25 | ALogp: | 2.5 |
| HBD: | 0 | HBA: | 3 |
| Rotatable Bonds: | 5 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 27.7 | Aromatic Rings: | 1 |
| Heavy Atoms: | 15 | QED Weighted: | 0.695 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.41 | MDCK Permeability: | 0.00002090 |
| Pgp-inhibitor: | 0.06 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.017 |
| 30% Bioavailability (F30%): | 0.067 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.511 | Plasma Protein Binding (PPB): | 84.89% |
| Volume Distribution (VD): | 0.92 | Fu: | 8.35% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.932 | CYP1A2-substrate: | 0.954 |
| CYP2C19-inhibitor: | 0.643 | CYP2C19-substrate: | 0.913 |
| CYP2C9-inhibitor: | 0.161 | CYP2C9-substrate: | 0.845 |
| CYP2D6-inhibitor: | 0.183 | CYP2D6-substrate: | 0.925 |
| CYP3A4-inhibitor: | 0.528 | CYP3A4-substrate: | 0.676 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 10.639 | Half-life (T1/2): | 0.856 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.035 | Human Hepatotoxicity (H-HT): | 0.071 |
| Drug-inuced Liver Injury (DILI): | 0.068 | AMES Toxicity: | 0.031 |
| Rat Oral Acute Toxicity: | 0.031 | Maximum Recommended Daily Dose: | 0.107 |
| Skin Sensitization: | 0.84 | Carcinogencity: | 0.543 |
| Eye Corrosion: | 0.21 | Eye Irritation: | 0.729 |
| Respiratory Toxicity: | 0.117 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001396 |  |
0.458 | D0AO5H |  |
0.500 | ||
| ENC001376 |  |
0.453 | D0A8FB |  |
0.352 | ||
| ENC001577 | 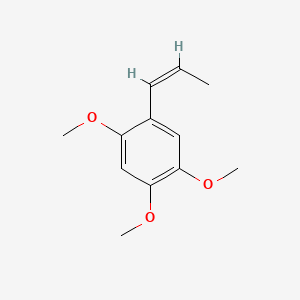 |
0.439 | D0Y7TS |  |
0.349 | ||
| ENC001410 | 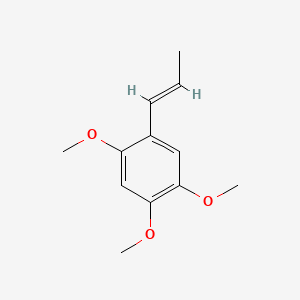 |
0.439 | D06QKV | 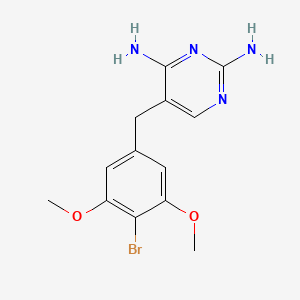 |
0.333 | ||
| ENC000095 |  |
0.423 | D0Q4YI | 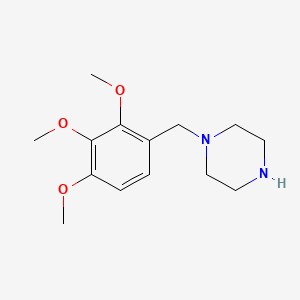 |
0.319 | ||
| ENC005523 | 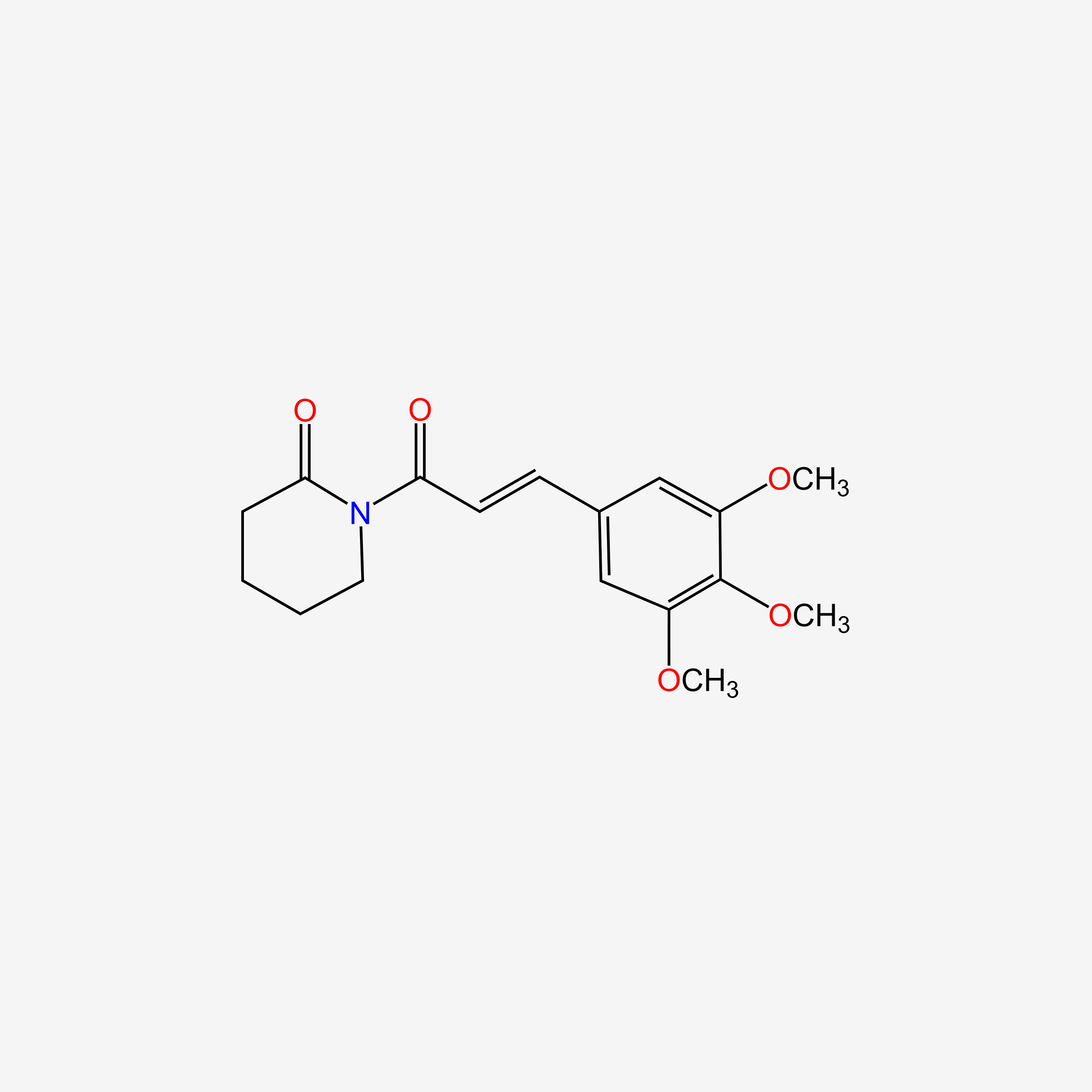 |
0.400 | D0NJ3V | 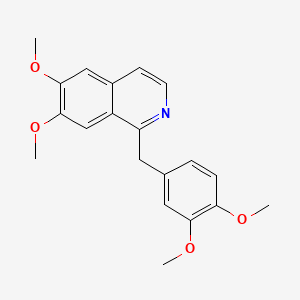 |
0.318 | ||
| ENC001423 | 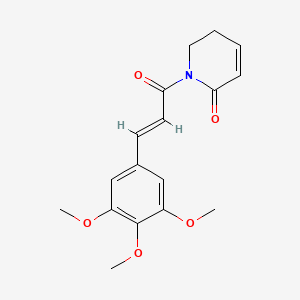 |
0.400 | D06GCK | 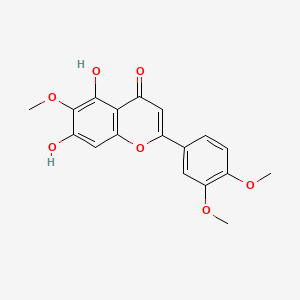 |
0.310 | ||
| ENC000304 |  |
0.382 | D02LZB |  |
0.303 | ||
| ENC001461 |  |
0.351 | D0D4HN |  |
0.299 | ||
| ENC000812 |  |
0.350 | D01FFA |  |
0.292 | ||