NPs Basic Information
|
Name |
Ethyl decanoate
|
| Molecular Formula | C12H24O2 | |
| IUPAC Name* |
ethyl decanoate
|
|
| SMILES |
CCCCCCCCCC(=O)OCC
|
|
| InChI |
InChI=1S/C12H24O2/c1-3-5-6-7-8-9-10-11-12(13)14-4-2/h3-11H2,1-2H3
|
|
| InChIKey |
RGXWDWUGBIJHDO-UHFFFAOYSA-N
|
|
| Synonyms |
ETHYL DECANOATE; Ethyl caprate; 110-38-3; Ethyl decylate; Decanoic acid, ethyl ester; Capric acid ethyl ester; Ethyl caprinate; Decanoic Acid Ethyl Ester; Ethyl n-decanoate; Capric acid, ethyl ester; FEMA No. 2432; NSC 8909; Decanoic acid-ethyl ester; n-Capric acid ethyl ester; GY39FB86UO; Ethyl ester of Decanoic acid; CHEBI:87430; NSC-8909; WE(2:0/10:0); Ethyl decanoate (natural); EINECS 203-761-9; UNII-GY39FB86UO; BRN 1762128; AI3-01976; Decanoic acid ethyl; MFCD00009581; ETHYL CAPRATE [MI]; DSSTox_CID_24363; DSSTox_RID_80171; DSSTox_GSID_44363; ETHYL CAPRATE [INCI]; WLN: 9VO2; ETHYL DECANOATE [FCC]; SCHEMBL116995; ETHYL DECANOATE [FHFI]; CHEMBL3184829; DTXSID0044363; FEMA 2432; NSC8909; ZINC1648324; Ethyl decanoate, analytical standard; Tox21_301180; LMFA07010455; AKOS009158697; Ethyl decanoate, >=98%, FCC, FG; CS-W015600; NCGC00248319-01; NCGC00255078-01; BS-14328; CAS-110-38-3; Ethyl decanoate, ReagentPlus(R), >=99%; D0022; FT-0626169; Ethyl decanoate, natural, >=98%, FCC, FG; E83014; Ethyl decanoate, Vetec(TM) reagent grade, 98%; A802183; Q5404454; W-108688; METHYL2-([4-HYDROXYDIHYDRO-2(3H)-ISOXAZOLYL]CARBONYL)BENZENECARBOXYLATE
|
|
| CAS | 110-38-3 | |
| PubChem CID | 8048 | |
| ChEMBL ID | CHEMBL3184829 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 200.32 | ALogp: | 4.6 |
| HBD: | 0 | HBA: | 2 |
| Rotatable Bonds: | 10 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 26.3 | Aromatic Rings: | 0 |
| Heavy Atoms: | 14 | QED Weighted: | 0.407 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.478 | MDCK Permeability: | 0.00002530 |
| Pgp-inhibitor: | 0.824 | Pgp-substrate: | 0.002 |
| Human Intestinal Absorption (HIA): | 0.001 | 20% Bioavailability (F20%): | 0.935 |
| 30% Bioavailability (F30%): | 0.98 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.773 | Plasma Protein Binding (PPB): | 94.97% |
| Volume Distribution (VD): | 0.698 | Fu: | 4.50% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.972 | CYP1A2-substrate: | 0.355 |
| CYP2C19-inhibitor: | 0.776 | CYP2C19-substrate: | 0.204 |
| CYP2C9-inhibitor: | 0.645 | CYP2C9-substrate: | 0.867 |
| CYP2D6-inhibitor: | 0.074 | CYP2D6-substrate: | 0.087 |
| CYP3A4-inhibitor: | 0.278 | CYP3A4-substrate: | 0.139 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.247 | Half-life (T1/2): | 0.631 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.106 | Human Hepatotoxicity (H-HT): | 0.018 |
| Drug-inuced Liver Injury (DILI): | 0.126 | AMES Toxicity: | 0.006 |
| Rat Oral Acute Toxicity: | 0.059 | Maximum Recommended Daily Dose: | 0.013 |
| Skin Sensitization: | 0.931 | Carcinogencity: | 0.132 |
| Eye Corrosion: | 0.97 | Eye Irritation: | 0.982 |
| Respiratory Toxicity: | 0.636 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000249 |  |
0.744 | D0G2KD |  |
0.530 | ||
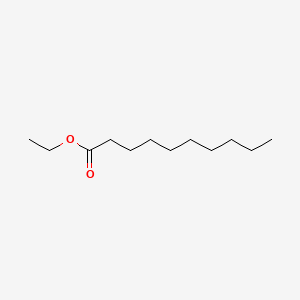
| ENC000742 | 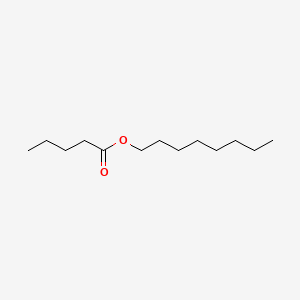 |
0.723 | D0AY9Q |  |
0.446 | ||
| ENC000487 | 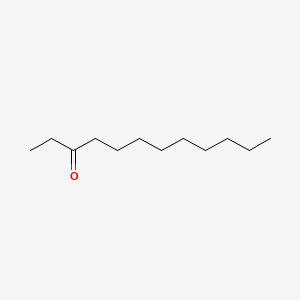 |
0.705 | D0Z5BC |  |
0.442 | ||
| ENC000260 | 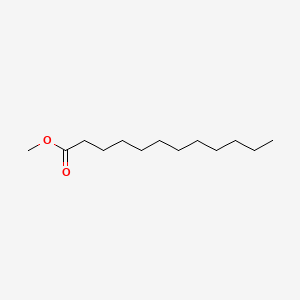 |
0.688 | D05ATI | 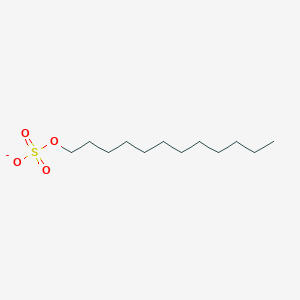 |
0.433 | ||
| ENC000419 |  |
0.684 | D03ZJE | 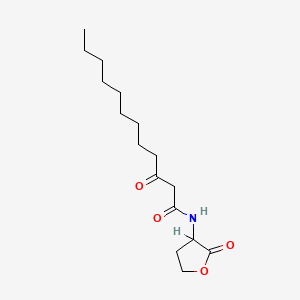 |
0.420 | ||
| ENC000265 |  |
0.674 | D07ILQ |  |
0.414 | ||
| ENC000556 | 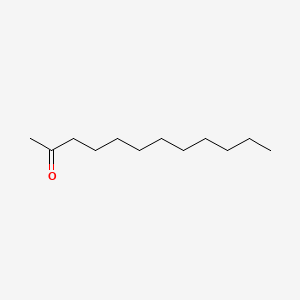 |
0.667 | D0XN8C |  |
0.400 | ||
| ENC000575 |  |
0.650 | D00MLW | 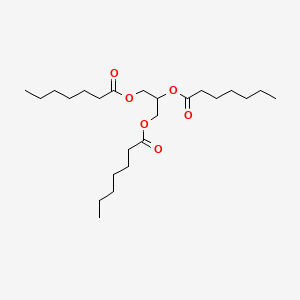 |
0.398 | ||
| ENC001313 |  |
0.648 | D0Z5SM | 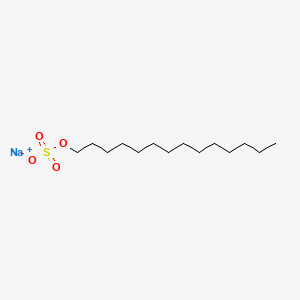 |
0.388 | ||
| ENC000495 | 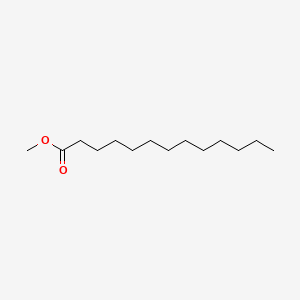 |
0.647 | D0O1PH |  |
0.382 | ||