NPs Basic Information
|
Name |
Methyl tridecanoate
|
| Molecular Formula | C14H28O2 | |
| IUPAC Name* |
methyl tridecanoate
|
|
| SMILES |
CCCCCCCCCCCCC(=O)OC
|
|
| InChI |
InChI=1S/C14H28O2/c1-3-4-5-6-7-8-9-10-11-12-13-14(15)16-2/h3-13H2,1-2H3
|
|
| InChIKey |
JNDDPBOKWCBQSM-UHFFFAOYSA-N
|
|
| Synonyms |
METHYL TRIDECANOATE; 1731-88-0; Tridecanoic acid, methyl ester; Tridecanoic acid methyl ester; Methyl n-tridecanoate; 61788-59-8; MFCD00008977; O2H463RING; n-Tridecanoic acid methyl ester; NSC-163375; 67762-40-7; EINECS 217-054-8; UNII-O2H463RING; NSC 163375; BRN 1769695; Tridecanoic acid,methyl ester; methyl tridecylate; Tridecanoic acid methyl; Methyl tridecanoate ester; tridecanoic acid-methyl ester; 4-02-00-01118 (Beilstein Handbook Reference); QSPL 203; SCHEMBL1647268; DTXSID8061923; Methyl ester of tridecanoic acid; CDAA-251013M; CHEBI:143578; BAA73188; ZINC1635630; EINECS 267-018-0; Methyl tridecanoate, >=97% (GC); NSC163375; AKOS015839775; CS-W004287; HY-W004287; Methyl tridecanoate, analytical standard; AS-60387; SY051771; DB-043928; FT-0633801; T0960; H10828; A881801; J-010886; Q24764359; EF80D759-D71D-495A-A128-F98E5A2D6415; 6-OXO-9ALPHA,11ALPHA,15S-TRIHYDROXY-PROSTA-13E,17Z-DIEN-1-OICACID
|
|
| CAS | 1731-88-0 | |
| PubChem CID | 15608 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 228.37 | ALogp: | 6.3 |
| HBD: | 0 | HBA: | 2 |
| Rotatable Bonds: | 12 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 26.3 | Aromatic Rings: | 0 |
| Heavy Atoms: | 16 | QED Weighted: | 0.368 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.637 | MDCK Permeability: | 0.00001990 |
| Pgp-inhibitor: | 0.27 | Pgp-substrate: | 0.002 |
| Human Intestinal Absorption (HIA): | 0.003 | 20% Bioavailability (F20%): | 0.986 |
| 30% Bioavailability (F30%): | 0.99 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.882 | Plasma Protein Binding (PPB): | 96.57% |
| Volume Distribution (VD): | 1.029 | Fu: | 2.42% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.926 | CYP1A2-substrate: | 0.362 |
| CYP2C19-inhibitor: | 0.616 | CYP2C19-substrate: | 0.252 |
| CYP2C9-inhibitor: | 0.391 | CYP2C9-substrate: | 0.927 |
| CYP2D6-inhibitor: | 0.061 | CYP2D6-substrate: | 0.091 |
| CYP3A4-inhibitor: | 0.38 | CYP3A4-substrate: | 0.094 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 5.91 | Half-life (T1/2): | 0.483 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.138 | Human Hepatotoxicity (H-HT): | 0.028 |
| Drug-inuced Liver Injury (DILI): | 0.262 | AMES Toxicity: | 0.006 |
| Rat Oral Acute Toxicity: | 0.031 | Maximum Recommended Daily Dose: | 0.02 |
| Skin Sensitization: | 0.946 | Carcinogencity: | 0.095 |
| Eye Corrosion: | 0.956 | Eye Irritation: | 0.975 |
| Respiratory Toxicity: | 0.876 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
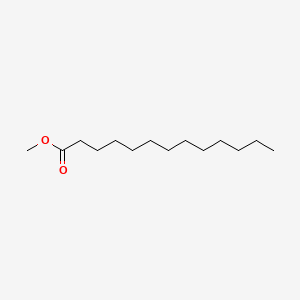
| ENC000604 |  |
0.938 | D05ATI | 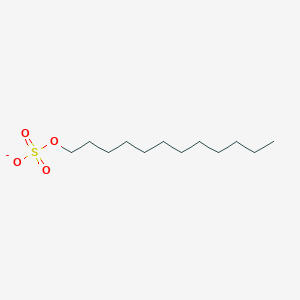 |
0.586 | ||
| ENC000260 | 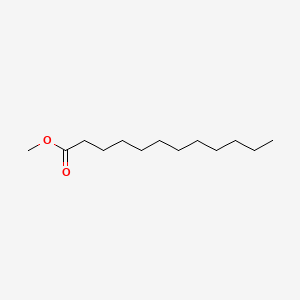 |
0.933 | D07ILQ |  |
0.544 | ||
| ENC000560 | 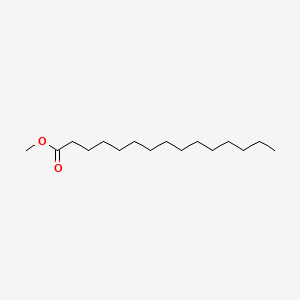 |
0.882 | D0Z5SM | 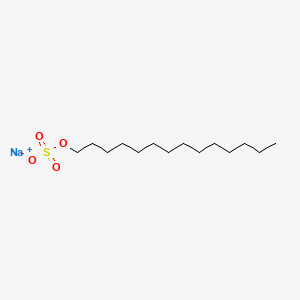 |
0.523 | ||
| ENC000271 |  |
0.833 | D0O1PH |  |
0.480 | ||
| ENC000249 |  |
0.800 | D0XN8C |  |
0.425 | ||
| ENC000496 | 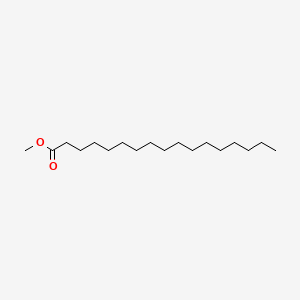 |
0.789 | D00FGR |  |
0.412 | ||
| ENC001519 | 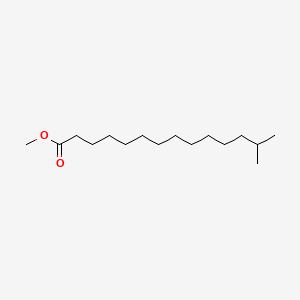 |
0.759 | D00MLW | 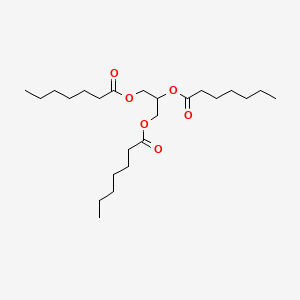 |
0.402 | ||
| ENC000280 |  |
0.750 | D09ANG |  |
0.400 | ||
| ENC001377 |  |
0.741 | D00AOJ |  |
0.400 | ||
| ENC000642 |  |
0.731 | D0Z5BC |  |
0.397 | ||