NPs Basic Information

|
Name |
Chloroxylenol
|
| Molecular Formula | C8H9ClO | |
| IUPAC Name* |
4-chloro-3,5-dimethylphenol
|
|
| SMILES |
CC1=CC(=CC(=C1Cl)C)O
|
|
| InChI |
InChI=1S/C8H9ClO/c1-5-3-7(10)4-6(2)8(5)9/h3-4,10H,1-2H3
|
|
| InChIKey |
OSDLLIBGSJNGJE-UHFFFAOYSA-N
|
|
| Synonyms |
chloroxylenol; 4-Chloro-3,5-dimethylphenol; 88-04-0; Dettol; 4-Chloro-3,5-xylenol; PCMX; p-Chloro-m-xylenol; Benzytol; 2-Chloro-m-xylenol; 4-Chloro-m-xylenol; Phenol, 4-chloro-3,5-dimethyl-; Ottasept; Desson; Espadol; Chloro-xylenol; parachlorometaxylenol; Ottasept Extra; Husept Extra; p-Chloro-3,5-xylenol; Willenol V; 3,5-Dimethyl-4-chlorophenol; Septiderm-Hydrochloride; Chloroxylenolum; Cloroxilenol; 2-Chloro-5-hydroxy-m-xylene; Dettol, liquid antiseptic; Nipacide MX; Parametaxylenol; RBA 777; 2-Chloro-5-hydroxy-1,3-dimethylbenzene; 4-Chloro-1-hydroxy-3,5-dimethylbenzene; 3,5-Xylenol, 4-chloro-; NSC 4971; Parachlorometoxylenol; 4-chloro-3,5-dimethyl-phenol; NSC-4971; 4-Chloro-3, 5-xylenol; Chlorxylenolum; CHEBI:34393; NSC4971; 0F32U78V2Q; 4-Chloro-3,5-dimethylphenol;PCMX; NCGC00094614-03; Clorossilenolo; DSSTox_CID_12316; DSSTox_RID_78913; DSSTox_GSID_32316; Caswell No. 218; Clorossilenolo [DCIT]; Vionexus; CAS-88-04-0; Cloroxilenol [INN-Spanish]; Camel (pesticide); Chloroxylenolum [INN-Latin]; HSDB 7427; EINECS 201-793-8; EPA Pesticide Chemical Code 086801; BRN 1862539; Ayrtol; UNII-0F32U78V2Q; AI3-08632; 5-dimethylphenol; Nipacide PX; Chloroxylenol(USAN; Chloroxylenol [USAN:USP:INN:BAN]; Chloroxylenol-[d6]; Spectrum_000138; 3, 4-chloro-; m-Xylenol, 4-chloro-; Para-chloro-meta-xylenol; Spectrum2_000136; Spectrum3_000344; Spectrum4_000281; Spectrum5_000713; Chloroxylenol (USP/INN); CHLOROXYLENOL [II]; CHLOROXYLENOL [MI]; CHLOROXYLENOL [INN]; 4-chloro-3,5dimethylphenol; CHLOROXYLENOL [HSDB]; CHLOROXYLENOL [INCI]; CHLOROXYLENOL [USAN]; SCHEMBL34163; BSPBio_002007; CHLOROXYLENOL [VANDF]; KBioGR_000802; KBioSS_000598; p-Chloro-3,5-dimethylphenol; MLS000028592; BIDD:ER0218; CHLOROXYLENOL [MART.]; DivK1c_000801; SPECTRUM1500182; SPBio_000212; WLN: QR DG C1 E1; CHLOROXYLENOL [USP-RS]; CHLOROXYLENOL [WHO-DD]; 3, 5-Dimethyl-4-chlorophenol; CHEMBL398440; ZINC1132; DTXSID0032316; HMS502I03; KBio1_000801; KBio2_000598; KBio2_003166; KBio2_005734; KBio3_001227; NINDS_000801; HMS1920K19; HMS2091C22; HMS2233N06; HMS3369I18; Para Chloro Meta Xylenol (PCMX); Pharmakon1600-01500182; 4-Chloro-3,5-dimethylphenol purum; HY-B1414; PARACHLOROMETOXYLENOL [VANDF]; Tox21_111305; Tox21_302047; AC-265; CCG-38943; CHLOROXYLENOL [USP MONOGRAPH]; MFCD00002324; NSC756683; s4518; STL183324; 4-Chloro-3,5-dimethylphenol, 99%; AKOS009159132; Tox21_111305_1; CS-4912; DB11121; NSC-756683; IDI1_000801; NCGC00094614-01; NCGC00094614-02; NCGC00094614-04; NCGC00094614-06; NCGC00094614-07; NCGC00255257-01; LS-13415; SMR000059157; SBI-0051310.P003; DB-028803; FT-0618059; EN300-39884; A16004; D03473; AB00051942_07; A842444; Q426460; SR-01000778359; SR-01000778359-2; 4-Chloro-3,5-dimethylphenol, purum, >=98.0% (T); 4-Chloro-3,5-xylenol, 4-Chloro-sym-m-xylenol, PCMX; BRD-K17223896-001-02-7; BRD-K17223896-001-06-8; F0001-2183; Z405702410; Chloroxylenol, British Pharmacopoeia (BP) Reference Standard; Chloroxylenol, United States Pharmacopeia (USP) Reference Standard; Chloroxylenol, Pharmaceutical Secondary Standard; Certified Reference Material
|
|
| CAS | 88-04-0 | |
| PubChem CID | 2723 | |
| ChEMBL ID | CHEMBL398440 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 156.61 | ALogp: | 2.0 |
| HBD: | 1 | HBA: | 1 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 20.2 | Aromatic Rings: | 1 |
| Heavy Atoms: | 10 | QED Weighted: | 0.61 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.456 | MDCK Permeability: | 0.00002400 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.014 |
| Human Intestinal Absorption (HIA): | 0.004 | 20% Bioavailability (F20%): | 0.911 |
| 30% Bioavailability (F30%): | 0.883 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.884 | Plasma Protein Binding (PPB): | 93.25% |
| Volume Distribution (VD): | 1.207 | Fu: | 7.00% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.963 | CYP1A2-substrate: | 0.938 |
| CYP2C19-inhibitor: | 0.866 | CYP2C19-substrate: | 0.542 |
| CYP2C9-inhibitor: | 0.304 | CYP2C9-substrate: | 0.921 |
| CYP2D6-inhibitor: | 0.708 | CYP2D6-substrate: | 0.895 |
| CYP3A4-inhibitor: | 0.509 | CYP3A4-substrate: | 0.317 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 12.322 | Half-life (T1/2): | 0.771 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.036 | Human Hepatotoxicity (H-HT): | 0.092 |
| Drug-inuced Liver Injury (DILI): | 0.2 | AMES Toxicity: | 0.02 |
| Rat Oral Acute Toxicity: | 0.052 | Maximum Recommended Daily Dose: | 0.896 |
| Skin Sensitization: | 0.674 | Carcinogencity: | 0.37 |
| Eye Corrosion: | 0.971 | Eye Irritation: | 0.993 |
| Respiratory Toxicity: | 0.796 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC002285 |  |
0.415 | D07EXH | 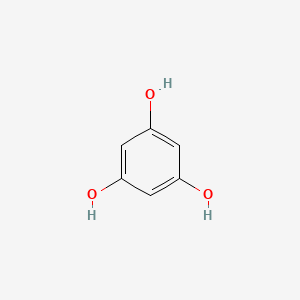 |
0.282 | ||
| ENC001026 |  |
0.410 | D0H2ZW | 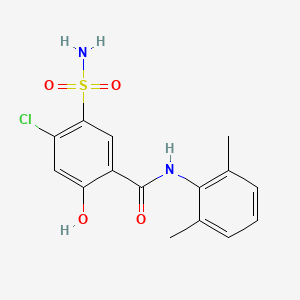 |
0.261 | ||
| ENC000729 | 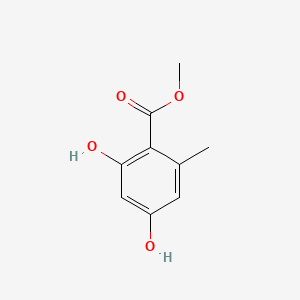 |
0.395 | D06GIP |  |
0.250 | ||
| ENC001617 |  |
0.391 | D02UFG | 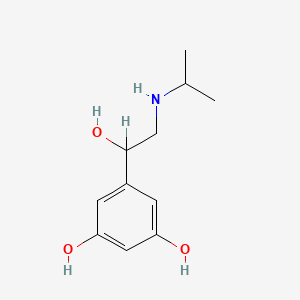 |
0.245 | ||
| ENC005178 |  |
0.391 | D0N0OU |  |
0.238 | ||
| ENC005704 |  |
0.390 | D0M8RC | 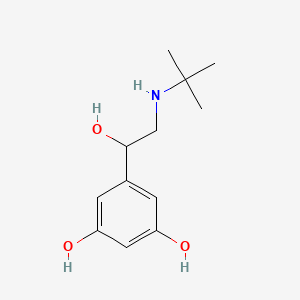 |
0.236 | ||
| ENC000674 | 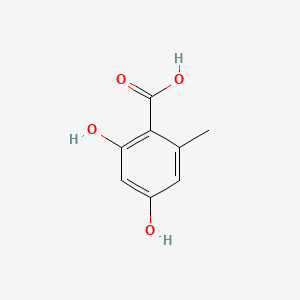 |
0.390 | D0Y4DY |  |
0.230 | ||
| ENC000614 |  |
0.389 | D0X5NX |  |
0.228 | ||
| ENC004013 |  |
0.378 | D0X0RI |  |
0.220 | ||
| ENC002405 | 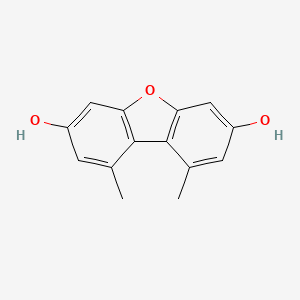 |
0.377 | D0FA2O |  |
0.217 | ||