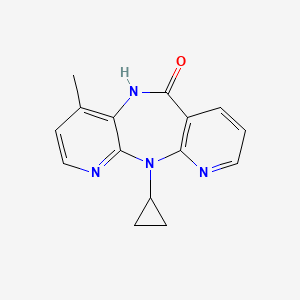
nevirapine, 129618-40-2, Viramune, BI-RG-587, Nevirapine anhydrous, 11-Cyclopropyl-4-methyl-5,11-dihydro-6H-dipyrido[3,2-b:2',3'-e][1,4]diazepin-6-one, NVP, BIRG 0587, BIRG587, Viramune XR, 11-Cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido(3,2-b:2',3'-e)(1,4)diazepin-6-one, Nevirapine, anhydrous, 11-CYCLOPROPYL-5,11-DIHYDRO-4-METHYL-6H-DIPYRIDO[3,2-B:2',3'-E][1,4]DIAZEPIN-6-ONE, Nevirapine teva, BI-RG 587, BIRG-0587, BIRG-587, CHEMBL57, 11-Cyclopropyl-4-methyl-5,11-dihydro-6H-dipyrido[2,3-e:3',2'-b][1,4]diazepin-6-one, 11-CYCLOPROPYL-4-METHYL-5H-DIPYRIDO[3,2-B:2',3'-E][1,4]DIAZEPIN-6(11H)-ONE, MFCD00866928, NEV, NSC-641530, 99DK7FVK1H, 6H-Dipyrido(3,2-b:2',3'-e)(1,4)diazepin-6-one, 11-cyclopropyl-5,11-dihydro-4-methyl-, MLS000084585, DTXSID7031797, CHEBI:63613, NSC641530, NCGC00065890-02, SMR000048458, DTXCID9010787, Viramune(TM), Viramune (TN), 2-cyclopropyl-7-methyl-2,4,9,15-tetraazatricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3,5,7,12,14-hexaen-10-one, CAS-129618-40-2, HSDB 7164, Nevirapine & CD4-IgG, Nevirapine & PRO 140, UNII-99DK7FVK1H, NSC 641530, Nevirapine (JAN/USP/INN), Nevirapine), 11-cyclopropyl-4-methyl-5H-dipyrido[[?],[?]][1,4]diazepin-6-one, BIRG 587, NON-NUCLEOSIDE RT INHIBITOR NEVIRAPINE, Viramune IR, 1vrt, 2hny, Nevirapine,(S), Nevirapine [USAN:USP:INN:BAN], BI-RG-587 & CD4-IgG, 6H-DIPYRIDO[3,2-B:2',3'-E][1,4]DIAZEPIN-6-ONE, 11-CYCLOPROPYL-5,11-DIHYDRO-4-METHYL-, Nevirapinum anhydrous, Nevirapine (Viramune), Nevirapine (anhydrous), NEVIRAPINE [MI], Opera_ID_934, NEVIRAPINE [INN], NEVIRAPINE [JAN], NEVIRAPINE [HSDB], NEVIRAPINE [USAN], NEVIRAPINE [VANDF], NEVIRAPINE [MART.], SCHEMBL3318, NEVIRAPINE [WHO-DD], MLS000759409, MLS001055309, MLS001201730, MLS001424058, MLS006011423, BIDD:GT0326, NEVIRAPINE [EMA EPAR], BDBM1434, BIRG0587, NEVIRAPINE [ORANGE BOOK], GTPL12676, NEVIRAPINE [EP MONOGRAPH], NEVIRAPINE [USP IMPURITY], Nevirapine for peak identification, HMS2051J09, HMS2231O23, HMS3264D21, HMS3371E03, HMS3393J09, HMS3655I08, HMS3715B10, NEVIRAPINE [USP MONOGRAPH], Pharmakon1600-01503842, ALBB-027264, BCP05587, Tox21 110982, Tox21_110982, Tox21_200770, AC-643, AC1280, BBL010768, NSC759902, STK580320, NEVIRAPINE ANHYDROUS [USP-RS], NEVIRAPINE ANHYDROUS [WHO-IP], AKOS005504351, Tox21_110982_1, AB07544, CCG-100939, DB00238, KS-5019, NC00189, NSC-759902, NCGC00065890-03, NCGC00065890-04, NCGC00065890-05, NCGC00065890-07, NCGC00065890-14, NCGC00258324-01, 11-Cyclopropyl-4-methyl-5,11-dihydro-6H-dipyrido[2,3-e:3',2'-b][1,4]diazepin-6-one & PRO 140 (Anti-CCR5 monoclonal antibody), 2-cyclopropyl-7-methyl-2,4,9,15-tetrazatricyclo[9.4.0.03,8]pentadeca-1(11),3,5,7,12,14-hexaen-10-one, HY-10570, N11-Cyclopropyl-4-methyl-5,11-dihydro-6H-dipyrido[3,2-b:2',3'-e]-[1,4]diazepin-6-one & CD4-immunoadhesin, Nevirapine 100 microg/mL in Acetonitrile, SY009679, BI-RG 587;NSC 641530;NVP, DB-041930, FT-0607215, FT-0672686, N0922, NEVIRAPINUM ANHYDROUS [WHO-IP LATIN], NS00004959, S1742, SW197569-2, C07263, D00435, EN300-119500, AB00393001-13, AB00393001-15, AB00393001_16, AB00393001_17, Q263713, F2173-0607, Z1521553473, BI-RG-587; BIRG 0587; BIRG587; HSDB 7164; NSC 641530; NVP, Nevirapine (anhydrous), European Pharmacopoeia (EP) Reference Standard, Nevirapine anhydrous, United States Pharmacopeia (USP) Reference Standard, Nevirapine, Pharmaceutical Secondary Standard; Certified Reference Material, 11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido-[3,2-b:2',3'-e][1,4]diazepin-6-one, 11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3,2-b :2',3'-e][1,4 ]diazepin-6-one, 11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3,2-b:2 ,3 -e][1,4]diazepin-6-one, 11-Cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3,2-b:2', 3'-e][1,4]diazepin-6-one, 6H-dipyrido[2,3-e:3',2'-b][1,4]diazepin-6-one, 11-cyclopropyl-5,11-dihydro-4-methyl-, Nevirapine for peak identification, European Pharmacopoeia (EP) Reference Standard, 11-Cyclopropyl-4-methyl-5,11-dihydro-6H-dipyrido[3,2-b:2 inverted exclamation mark ,3 inverted exclamation mark -e][1,4]diazepin-6-one, 2-cyclopropyl-7-methyl-2,4,9,15-tetraazatricyclo[9.4.0.0,3,8]pentadeca-1(15),3(8),4,6,11,13-hexaen-10-one, 2-cyclopropyl-7-methyl-2,4,9,15-tetraazatricyclo[9.4.0.0^{3,8}]pentadeca-1(15),3,5,7,11,13-hexaen-10-one