NPs Basic Information

|
Name |
Methyl 2-(4-(hydroxymethyl)phenyl)acetate
|
| Molecular Formula | C10H12O3 | |
| IUPAC Name* |
methyl 2-[4-(hydroxymethyl)phenyl]acetate
|
|
| SMILES |
COC(=O)CC1=CC=C(C=C1)CO
|
|
| InChI |
InChI=1S/C10H12O3/c1-13-10(12)6-8-2-4-9(7-11)5-3-8/h2-5,11H,6-7H2,1H3
|
|
| InChIKey |
LLDQUDYCTIKKFV-UHFFFAOYSA-N
|
|
| Synonyms |
methyl 2-(4-(hydroxymethyl)phenyl)acetate; 155380-11-3; METHYL 2-[4-(HYDROXYMETHYL)PHENYL]ACETATE; Benzeneacetic acid, 4-(hydroxymethyl)-, methyl ester; SCHEMBL112297; DTXSID80503105; methyl p-hydroxymethylphenylacetate; MFCD08061910; ZINC39249059; AKOS006287393; methyl [4-(hydroxymethyl)phenyl]acetate; methyl2-(4-(hydroxymethyl)phenyl)acetate; CS-0187457; FT-0774848; E77966; (4-hydroxymethyl-phenyl)-acetic acid methyl ester; A907332
|
|
| CAS | 155380-11-3 | |
| PubChem CID | 12575321 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 180.2 | ALogp: | 0.9 |
| HBD: | 1 | HBA: | 3 |
| Rotatable Bonds: | 4 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 46.5 | Aromatic Rings: | 1 |
| Heavy Atoms: | 13 | QED Weighted: | 0.713 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.345 | MDCK Permeability: | 0.00005260 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.056 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.002 |
| 30% Bioavailability (F30%): | 0.282 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.689 | Plasma Protein Binding (PPB): | 34.73% |
| Volume Distribution (VD): | 0.672 | Fu: | 64.73% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.458 | CYP1A2-substrate: | 0.358 |
| CYP2C19-inhibitor: | 0.531 | CYP2C19-substrate: | 0.646 |
| CYP2C9-inhibitor: | 0.113 | CYP2C9-substrate: | 0.281 |
| CYP2D6-inhibitor: | 0.019 | CYP2D6-substrate: | 0.532 |
| CYP3A4-inhibitor: | 0.041 | CYP3A4-substrate: | 0.507 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 9.21 | Half-life (T1/2): | 0.894 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.039 | Human Hepatotoxicity (H-HT): | 0.155 |
| Drug-inuced Liver Injury (DILI): | 0.836 | AMES Toxicity: | 0.059 |
| Rat Oral Acute Toxicity: | 0.047 | Maximum Recommended Daily Dose: | 0.011 |
| Skin Sensitization: | 0.279 | Carcinogencity: | 0.225 |
| Eye Corrosion: | 0.005 | Eye Irritation: | 0.708 |
| Respiratory Toxicity: | 0.028 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC004860 |  |
0.683 | D02HXS |  |
0.397 | ||
| ENC000223 | 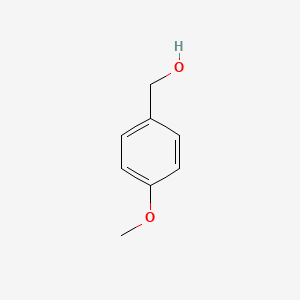 |
0.524 | D02AQY |  |
0.396 | ||
| ENC000208 |  |
0.489 | D03XTC |  |
0.362 | ||
| ENC002095 |  |
0.479 | D01UXC | 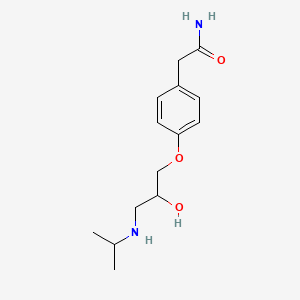 |
0.354 | ||
| ENC001338 | 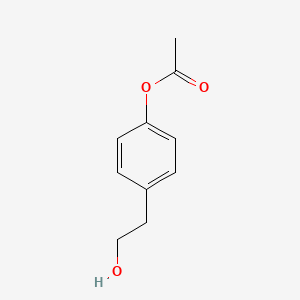 |
0.440 | D0B3QM |  |
0.352 | ||
| ENC000774 | 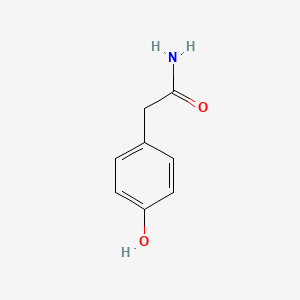 |
0.435 | D01CRB |  |
0.340 | ||
| ENC000006 |  |
0.435 | D0R1QE |  |
0.333 | ||
| ENC000222 | 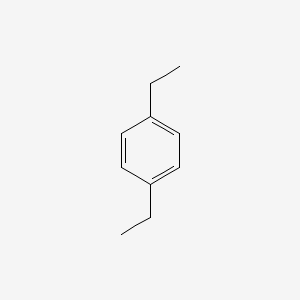 |
0.422 | D0Y7EM | 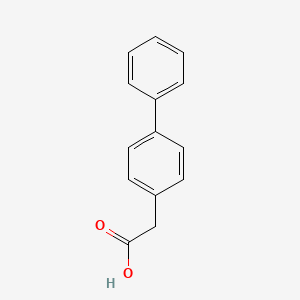 |
0.323 | ||
| ENC001422 |  |
0.412 | D0W1RY |  |
0.314 | ||
| ENC002602 | 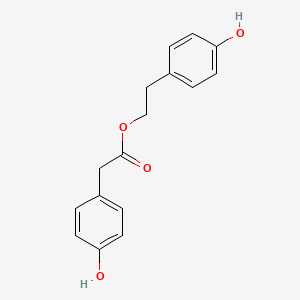 |
0.409 | D05CKR |  |
0.313 | ||