NPs Basic Information
|
Name |
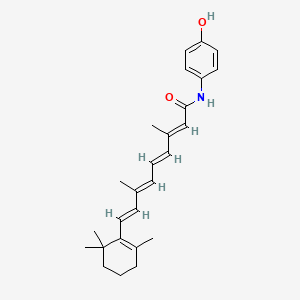
Fenretinide
|
| Molecular Formula | C26H33NO2 | |
| IUPAC Name* |
(2E,4E,6E,8E)-N-(4-hydroxyphenyl)-3,7-dimethyl-9-(2,6,6-trimethylcyclohexen-1-yl)nona-2,4,6,8-tetraenamide
|
|
| SMILES |
CC1=C(C(CCC1)(C)C)/C=C/C(=C/C=C/C(=C/C(=O)NC2=CC=C(C=C2)O)/C)/C
|
|
| InChI |
InChI=1S/C26H33NO2/c1-19(11-16-24-21(3)10-7-17-26(24,4)5)8-6-9-20(2)18-25(29)27-22-12-14-23(28)15-13-22/h6,8-9,11-16,18,28H,7,10,17H2,1-5H3,(H,27,29)/b9-6+,16-11+,19-8+,20-18+
|
|
| InChIKey |
AKJHMTWEGVYYSE-FXILSDISSA-N
|
|
| Synonyms |
FENRETINIDE; 65646-68-6; N-(4-Hydroxyphenyl)retinamide; 4-HPR; 4-hydroxyphenylretinamide; 4-Hydroxyphenyl retinamide; Retinoic acid p-hydroxyanilide; all-trans-4'-Hydroxyretinanilide; McN-R-1967; Fenretinida; Fenretinidum; 4-hydroxy(phenyl)retinamide; N-(4-Hydroxyphenyl)all-Trans Retinamide; Retinamide, N-(4-hydroxyphenyl)-; Rii retinamide; Retinoic acid p-hydroxyphenylamide; 4-(hydroxyphenyl)retinamide; 15-[(4-hydroxyphenyl)amino]retinal; (2E,4E,6E,8E)-N-(4-hydroxyphenyl)-3,7-dimethyl-9-(2,6,6-trimethylcyclohex-1-en-1-yl)nona-2,4,6,8-tetraenamide; NSC-760419; 187EJ7QEXL; CHEMBL7301; MLS002701698; CHEBI:42588; NSC-374551; NCGC00090752-03; DSSTox_CID_12005; DSSTox_RID_78900; DSSTox_GSID_32005; Fenretinidum [Latin]; Fenretinida [Spanish]; 4HPR; Fenretinide [USAN:INN]; SMR001456303; CAS-65646-68-6; CCRIS 3260; SR-01000075917; (2E,4E,6E,8E)-N-(4-hydroxyphenyl)-3,7-dimethyl-9-(2,6,6-trimethylcyclohexen-1-yl)nona-2,4,6,8-tetraenamide; MFCD00792674; UNII-187EJ7QEXL; BRN 5769490; SYT-101; ST-602; Fenretinide; 4-HPR; Fenretinide (4-HPR); p-Hydroxyphenylretinamide; FENRETINIDE [MI]; Spectrum5_001939; FENRETINIDE [INN]; Fenretinide (USAN/INN); FENRETINIDE [USAN]; FENRETINIDE [VANDF]; FENRETINIDE [MART.]; Lopac0_000625; SCHEMBL11703; SCHEMBL11704; BSPBio_001419; FENRETINIDE [WHO-DD]; MLS001055399; MLS006010811; BML2-E08; N-(4-hydroxyphenyl)-retinamide; DTXSID2032005; SCHEMBL15703189; CHEBI:92493; AMY9087; 15-(4-Hydroxyanilino)retinal #; HMS1361G21; HMS1791G21; HMS1989G21; HMS2089B17; HMS3261N12; HMS3402G21; HMS3412M06; HMS3676M06; Pharmakon1600-01505602; 4-HPR;(4-Hydroxyphenyl)retinamide; BCP06908; EX-A4102; N-(4-hydroxyphenyl)-3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexenyl)nona-2,4,6,8-tetraenamide; ZINC3871023; Tox21_111007; Tox21_200989; Tox21_500625; 1-enyl)nona-2,4,6,8-tetraenamide; BDBM50092055; HSCI1_000112; NSC374551; NSC760419; s5233; CALIX[4!-BIS-CROWN-6,95; AKOS024456572; Tox21_111007_1; CCG-204713; CS-0789; DB05076; LP00625; MK-4016; N-(4-hydroxyphenyl)retinamide, 4-HPR; NSC 760419; SDCCGSBI-0050606.P002; all-trans-n-(4-hydroxyphenyl)retinamide; dimethyl-9-(2,6,6-trimethylcyclohex-; IDI1_033889; Retinoic acid p-hydroxyanilide, >=95%; NCGC00090752-01; NCGC00090752-02; NCGC00090752-04; NCGC00090752-05; NCGC00090752-06; NCGC00090752-07; NCGC00090752-09; NCGC00090752-10; NCGC00090752-11; NCGC00090752-12; NCGC00090752-20; NCGC00258542-01; NCGC00261310-01; AS-59667; BP-13369; HY-15373; SMR000677938; EU-0100625; H1464; D04162; H 7779; (2E,4E,6E,8E)-N-(4-hydroxyphenyl)-3,7-; AB00172992-07; 646F686; A835178; Q5443576; SR-01000075917-1; SR-01000075917-4; N-(4-HYDROXYPHENYL)-ALL-TRANS-VITAMIN A AMIDE; (2E,4E,6E,8E)-3,7-Dimethyl-9-(2,6,6-trimethyl-cyclohex-1-enyl)-nona-2,4,6,8-tetraenoic acid (4-hydroxy-phenyl)-amide; (2E,4E,6E,8E)-N-(4-Hydroxyphenyl)-3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexenyl)nona-2,4,6,8-tetraenamide; (2E,4E,6E,8E)-N-(4-hydroxyphenyl)-3,7-dimethyl-9-(2,6,6-trimethylcyclohex-1-enyl)nona-2,4,6,8-tetraenamide; (2E,4E,6E,8E)-N-(4-hydroxyphenyl)-3,7-dimethyl-9-(2,6,6-trimethylcyclohexen-1-yl)nona-2,4,6,8-tetraenamide;Fenretinide; 2,6,8-Nonatetraenamide, N-(4-hydroxyphenyl)-3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl-, (all-E)-; 3,7-Dimethyl-9-(2,6,6-trimethyl-cyclohex-1-enyl)-nona-2,4,6,8-tetraenoic acid (4-hydroxy-phenyl)-amide; N-(4-Hydroxyphenyl)-3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexenyl)-2,4,6,8-nonatetraeneamide
|
|
| CAS | 65646-68-6 | |
| PubChem CID | 5288209 | |
| ChEMBL ID | CHEMBL7301 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 391.5 | ALogp: | 7.3 |
| HBD: | 2 | HBA: | 2 |
| Rotatable Bonds: | 6 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 49.3 | Aromatic Rings: | 2 |
| Heavy Atoms: | 29 | QED Weighted: | 0.318 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.058 | MDCK Permeability: | 0.00002150 |
| Pgp-inhibitor: | 0.957 | Pgp-substrate: | 0.025 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.002 |
| 30% Bioavailability (F30%): | 0.001 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.021 | Plasma Protein Binding (PPB): | 100.02% |
| Volume Distribution (VD): | 2.978 | Fu: | 1.17% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.609 | CYP1A2-substrate: | 0.959 |
| CYP2C19-inhibitor: | 0.895 | CYP2C19-substrate: | 0.746 |
| CYP2C9-inhibitor: | 0.915 | CYP2C9-substrate: | 0.993 |
| CYP2D6-inhibitor: | 0.955 | CYP2D6-substrate: | 0.95 |
| CYP3A4-inhibitor: | 0.806 | CYP3A4-substrate: | 0.671 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 1.354 | Half-life (T1/2): | 0.688 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.748 | Human Hepatotoxicity (H-HT): | 0.372 |
| Drug-inuced Liver Injury (DILI): | 0.064 | AMES Toxicity: | 0.317 |
| Rat Oral Acute Toxicity: | 0.245 | Maximum Recommended Daily Dose: | 0.888 |
| Skin Sensitization: | 0.937 | Carcinogencity: | 0.619 |
| Eye Corrosion: | 0.003 | Eye Irritation: | 0.301 |
| Respiratory Toxicity: | 0.934 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001425 |  |
0.410 | D00DKK |  |
0.655 | ||
| ENC000072 |  |
0.325 | D02DGU |  |
0.655 | ||
| ENC000328 | 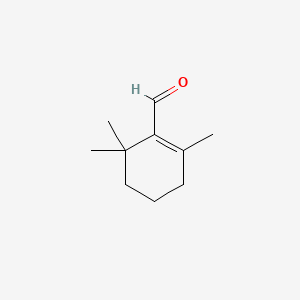 |
0.298 | D0G3PI | 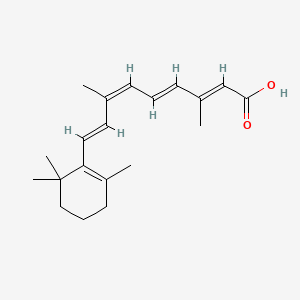 |
0.655 | ||
| ENC003853 | 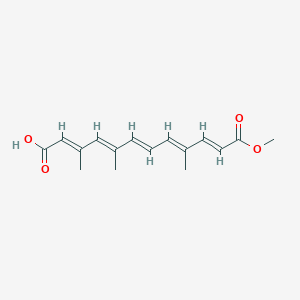 |
0.291 | D0S7WX | 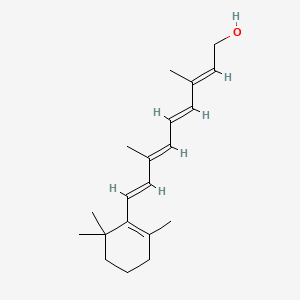 |
0.575 | ||
| ENC003854 |  |
0.291 | D0MY8N | 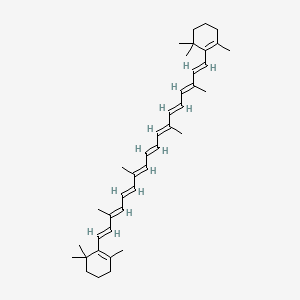 |
0.377 | ||
| ENC003852 | 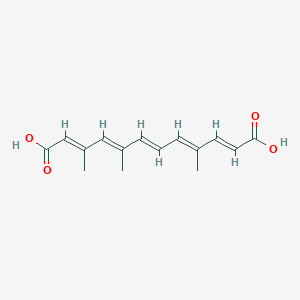 |
0.287 | D05QDC | 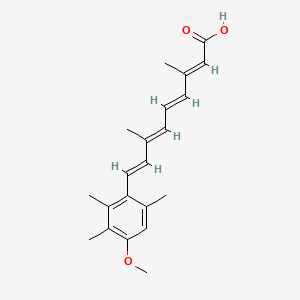 |
0.371 | ||
| ENC002787 | 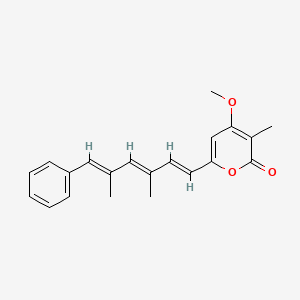 |
0.267 | D0B1IP | 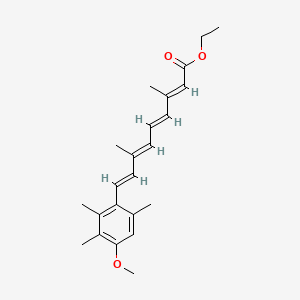 |
0.339 | ||
| ENC001738 | 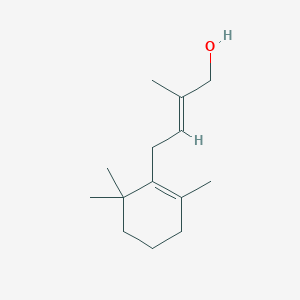 |
0.263 | D0U5QK |  |
0.325 | ||
| ENC001731 |  |
0.261 | D0N0RU |  |
0.250 | ||
| ENC001420 |  |
0.256 | D03VFL | 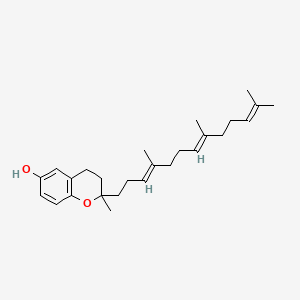 |
0.244 | ||