NPs Basic Information
|
Name |
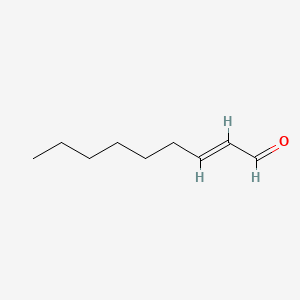
2-Nonenal
|
| Molecular Formula | C9H16O | |
| IUPAC Name* |
(E)-non-2-enal
|
|
| SMILES |
CCCCCC/C=C/C=O
|
|
| InChI |
InChI=1S/C9H16O/c1-2-3-4-5-6-7-8-9-10/h7-9H,2-6H2,1H3/b8-7+
|
|
| InChIKey |
BSAIUMLZVGUGKX-BQYQJAHWSA-N
|
|
| Synonyms |
trans-2-Nonenal; 2-NONENAL; 18829-56-6; (E)-Non-2-enal; (E)-2-Nonenal; 2-Nonenal, (2E)-; 2463-53-8; 2-Nonenal, (E)-; 2-trans-Nonenal; 3-Hexyl-2-propenal; trans-2-Nonen-1-al; Non-2-enal; (2E)-non-2-enal; 3-Hexylacrolein; (E)-2-nonen-1-al; (2E)-2-Nonenal; FEMA No. 3213; 2-Nonen-1-al; beta-Hexylacrolein; Heptylideneacetaldehyde; alpha-Nonenyl aldehyde; 8VEO649985; 3-hexyl-acrolein; .beta.-Hexylacrolein; trans-2-Nonenal (natural); trans-Non-2-enal; .alpha.-Nonenyl aldehyde; CCRIS 3326; CCRIS 9203; EINECS 219-562-5; EINECS 242-609-6; MFCD00007012; NSC 20746; CHEBI:61726; BRN 1722170; AI3-36268; UNII-8VEO649985; t-2-nonenal; (2e)-nonenal; trans-2-nonealdehyde; heptylidene acetaldehyde; trans-2-Nonenal, 97%; UNII-93C6BZW2TV; 2-NONENAL [FHFI]; 2-NONENAL [MI]; 2-NONENAL, TRANS-; 93C6BZW2TV; DSSTox_CID_27086; DSSTox_RID_82098; DSSTox_GSID_47086; 4-01-00-03502 (Beilstein Handbook Reference); SCHEMBL102464; CHEMBL450072; DTXSID0047086; (E)-2-NONENAL [FCC]; CHEBI:142592; trans-2-Nonenal, >=95%, FG; ZINC1571215; Tox21_300815; LMFA06000041; trans-2-Nonenal, analytical standard; AKOS015902295; NCGC00248181-01; NCGC00254719-01; AS-17376; LS-13683; CAS-18829-56-6; N0430; N0483; EN300-396736; EN300-1664833; J-012128; Q4596912
|
|
| CAS | 18829-56-6 | |
| PubChem CID | 5283335 | |
| ChEMBL ID | CHEMBL450072 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 140.22 | ALogp: | 3.1 |
| HBD: | 0 | HBA: | 1 |
| Rotatable Bonds: | 6 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 17.1 | Aromatic Rings: | 0 |
| Heavy Atoms: | 10 | QED Weighted: | 0.313 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.335 | MDCK Permeability: | 0.00002920 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.006 | 20% Bioavailability (F20%): | 0.332 |
| 30% Bioavailability (F30%): | 0.959 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.998 | Plasma Protein Binding (PPB): | 78.99% |
| Volume Distribution (VD): | 0.791 | Fu: | 25.71% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.943 | CYP1A2-substrate: | 0.781 |
| CYP2C19-inhibitor: | 0.627 | CYP2C19-substrate: | 0.823 |
| CYP2C9-inhibitor: | 0.259 | CYP2C9-substrate: | 0.957 |
| CYP2D6-inhibitor: | 0.067 | CYP2D6-substrate: | 0.865 |
| CYP3A4-inhibitor: | 0.048 | CYP3A4-substrate: | 0.16 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 4.055 | Half-life (T1/2): | 0.586 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.034 | Human Hepatotoxicity (H-HT): | 0.054 |
| Drug-inuced Liver Injury (DILI): | 0.088 | AMES Toxicity: | 0.643 |
| Rat Oral Acute Toxicity: | 0.078 | Maximum Recommended Daily Dose: | 0.058 |
| Skin Sensitization: | 0.952 | Carcinogencity: | 0.718 |
| Eye Corrosion: | 0.982 | Eye Irritation: | 0.988 |
| Respiratory Toxicity: | 0.937 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001599 |  |
0.903 | D0UE9X |  |
0.328 | ||
| ENC001597 |  |
0.893 | D0O1TC |  |
0.319 | ||
| ENC001601 | 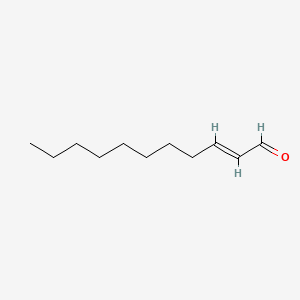 |
0.824 | D01QLH |  |
0.316 | ||
| ENC001654 |  |
0.724 | D0O1PH |  |
0.288 | ||
| ENC001724 |  |
0.711 | D0AY9Q |  |
0.273 | ||
| ENC001808 |  |
0.686 | D06FEA | 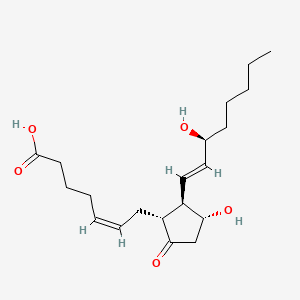 |
0.260 | ||
| ENC001600 |  |
0.686 | D0Z5BC |  |
0.255 | ||
| ENC001668 | 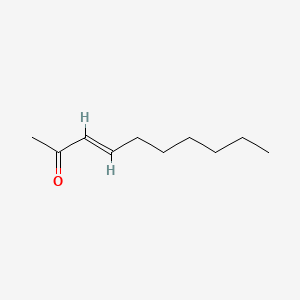 |
0.568 | D0OR6A |  |
0.253 | ||
| ENC000032 |  |
0.559 | D05ATI | 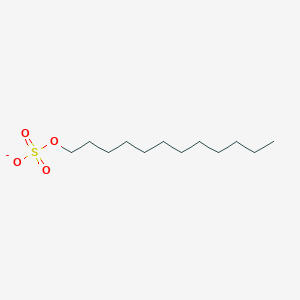 |
0.250 | ||
| ENC001587 | 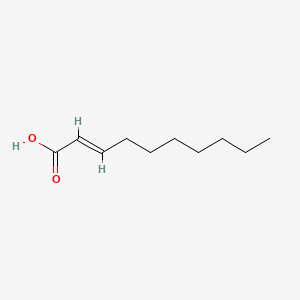 |
0.525 | D0N3NO | 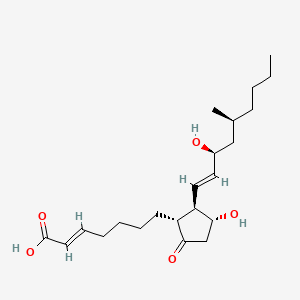 |
0.244 | ||