NPs Basic Information

|
Name |
4-Amino-1,2,5-oxadiazole-3-carbohydrazide
|
| Molecular Formula | C3H5N5O2 | |
| IUPAC Name* |
4-amino-1,2,5-oxadiazole-3-carbohydrazide
|
|
| SMILES |
C1(=NON=C1N)C(=O)NN
|
|
| InChI |
InChI=1S/C3H5N5O2/c4-2-1(3(9)6-5)7-10-8-2/h5H2,(H2,4,8)(H,6,9)
|
|
| InChIKey |
ASQCREDXLDLPDJ-UHFFFAOYSA-N
|
|
| Synonyms |
4-Amino-1,2,5-oxadiazole-3-carbohydrazide; 246048-72-6; 4-Amino-1,2,5-oxadiazol-3-carbohydrazide; 1,2,5-Oxadiazole-3-carboxylic acid, 4-amino-, hydrazide; Oprea1_397873; 3-aminofurazan-4-carbohydrazide; SCHEMBL18249667; DTXSID60337480; ALBB-010461; ZINC3882149; Furazan-3-carbohydrazide, 4-amino-; BBL028722; MFCD00508456; STK744321; AKOS000297342; VS-08927; CS-0240225; 4-Amino-1,2,5-oxadiazole-3-carbohydrazide #; EN300-226881
|
|
| CAS | 246048-72-6 | |
| PubChem CID | 543041 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 143.1 | ALogp: | -1.2 |
| HBD: | 3 | HBA: | 6 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 120.0 | Aromatic Rings: | 1 |
| Heavy Atoms: | 10 | QED Weighted: | 0.258 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.512 | MDCK Permeability: | 0.00000608 |
| Pgp-inhibitor: | 0.002 | Pgp-substrate: | 0.175 |
| Human Intestinal Absorption (HIA): | 0.011 | 20% Bioavailability (F20%): | 0.002 |
| 30% Bioavailability (F30%): | 0.009 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.983 | Plasma Protein Binding (PPB): | 10.99% |
| Volume Distribution (VD): | 0.836 | Fu: | 75.89% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.58 | CYP1A2-substrate: | 0.328 |
| CYP2C19-inhibitor: | 0.259 | CYP2C19-substrate: | 0.064 |
| CYP2C9-inhibitor: | 0.023 | CYP2C9-substrate: | 0.099 |
| CYP2D6-inhibitor: | 0.119 | CYP2D6-substrate: | 0.11 |
| CYP3A4-inhibitor: | 0.063 | CYP3A4-substrate: | 0.15 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 3.35 | Half-life (T1/2): | 0.891 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.008 | Human Hepatotoxicity (H-HT): | 0.981 |
| Drug-inuced Liver Injury (DILI): | 0.99 | AMES Toxicity: | 0.727 |
| Rat Oral Acute Toxicity: | 0.735 | Maximum Recommended Daily Dose: | 0.09 |
| Skin Sensitization: | 0.837 | Carcinogencity: | 0.965 |
| Eye Corrosion: | 0.098 | Eye Irritation: | 0.965 |
| Respiratory Toxicity: | 0.986 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000067 |  |
0.167 | D09XQF |  |
0.250 | ||
| ENC000352 |  |
0.167 | D0I0RJ | 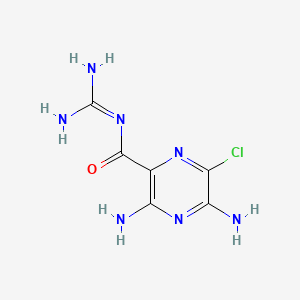 |
0.222 | ||
| ENC000650 | 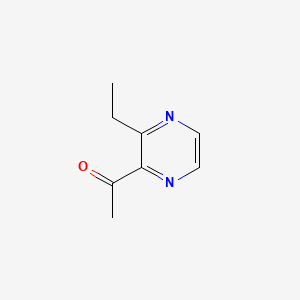 |
0.163 | D0E1SW | 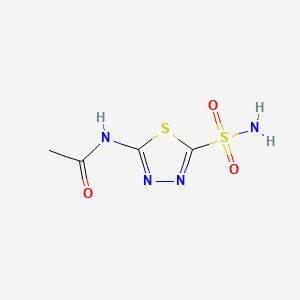 |
0.173 | ||
| ENC003710 | 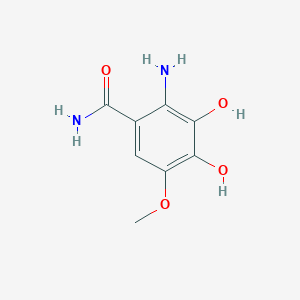 |
0.145 | D02XBW |  |
0.167 | ||
| ENC000111 | 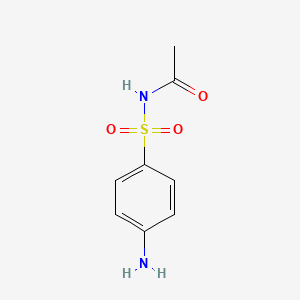 |
0.143 | D0C8EU |  |
0.161 | ||
| ENC000103 |  |
0.143 | D06OAV |  |
0.158 | ||
| ENC000011 |  |
0.143 | D0XF8W |  |
0.156 | ||
| ENC001108 |  |
0.143 | D07CWD | 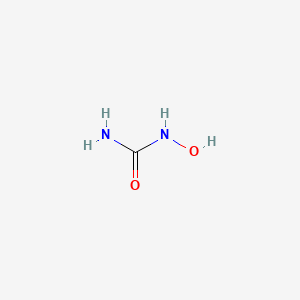 |
0.152 | ||
| ENC000467 |  |
0.140 | D0I2VK |  |
0.152 | ||
| ENC000303 |  |
0.140 | D01BQK | 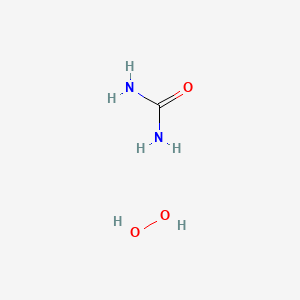 |
0.152 | ||