NPs Basic Information

|
Name |
Methyl anthranilate
|
| Molecular Formula | C8H9NO2 | |
| IUPAC Name* |
methyl 2-aminobenzoate
|
|
| SMILES |
COC(=O)C1=CC=CC=C1N
|
|
| InChI |
InChI=1S/C8H9NO2/c1-11-8(10)6-4-2-3-5-7(6)9/h2-5H,9H2,1H3
|
|
| InChIKey |
VAMXMNNIEUEQDV-UHFFFAOYSA-N
|
|
| Synonyms |
METHYL ANTHRANILATE; Methyl 2-aminobenzoate; 134-20-3; 2-Aminobenzoic acid methyl ester; Methyl o-aminobenzoate; Methylanthranilate; Anthranilic acid methyl ester; o-Carbomethoxyaniline; 2-Carbomethoxyaniline; 2-(Methoxycarbonyl)aniline; Benzoic acid, 2-amino-, methyl ester; Anthranilic acid, methyl ester; O-methyl anthranilate; FEMA No. 2682; o-Aminobenzoic acid methyl ester; methyl antranilate; Benzoic acid, amino-, methyl ester; NSC 3109; Methylester kyseliny anthranilove; o-Amino methyl benzoate; MFCD00007710; 2-Aminobenzoic acid-methyl ester; CHEBI:73244; o-Aminobenzoic acid, methyl ester; 2-amino-benzoic acid methyl ester; 981I0C1E5W; Methyl ester of o-Aminobenzoic acid; NSC-3109; Methyl 2-Aminobenzoate (Methyl Anthranilate); DSSTox_CID_5567; DSSTox_RID_77832; DSSTox_GSID_25567; Amino methyl benzoate, o-; CAS-134-20-3; Methyl anthranilate (natural); METHYL-2-AMINOBENZOATE; CCRIS 1349; HSDB 1008; EINECS 205-132-4; Epa Pesticide Chemical Code 128725; BRN 0606965; Methylester kyseliny anthranilove [Czech]; UNII-981I0C1E5W; AI3-01022; FEMA 2682; Carbomethoxyaniline; 2-Aminobenzoic acid, methyl ester; methyl aminobenzoate; Anthranilic acid methyl; o-methoxycarbonylaniline; methyl 2-amino-benzoate; WLN: ZR BVO1; Acetaminophen 100% Powder; SCHEMBL57713; Methyl 2-aminobenzoate, 99%; CHEMBL1493986; DTXSID6025567; METHYL ANTHRANILATE [MI]; METHYL ANTHRANILATE [FCC]; NSC3109; 2-amino benzoic acid methyl ester; Natural Mandarin Pettigrain F.D.; NATURAL METHYL ANTHRANILATE; METHYL ANTHRANILATE [FHFI]; METHYL ANTHRANILATE [HSDB]; METHYL ANTHRANILATE [INCI]; ZINC157525; METHYL ANTHRANILATE [VANDF]; METHYL ANTHRANILATE [MART.]; STR00871; Tox21_201657; Tox21_300347; METHYL ANTHRANILATE [WHO-DD]; methyl ester of o-amino benzoic acid; STK045541; AKOS000119222; AM10669; CS-W019645; Methyl anthranilate, >=98%, FCC, FG; NCGC00091409-01; NCGC00091409-02; NCGC00091409-03; NCGC00254347-01; NCGC00259206-01; AC-11600; BENZOIC ACID METHYL ESTER,2-AMINO; HY-77342; Methyl-2-aminobenzoate Methyl anthranilate; 5-NITRO-PYRIDINE-2-SULFONYLCHLORIDE; DB-042220; Methyl anthranilate, natural, >=99%, FG; A0500; FT-0622414; C20634; D77860; Methyl 2-aminobenzoate, ReagentPlus(R), >=99%; Methyl anthranilate, natural (US), >=99%, FG; Q420894; W-108288; F2141-0131; Methyl 2-aminobenzoate, Vetec(TM) reagent grade, 98%
|
|
| CAS | 134-20-3 | |
| PubChem CID | 8635 | |
| ChEMBL ID | CHEMBL1493986 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 151.16 | ALogp: | 1.9 |
| HBD: | 1 | HBA: | 3 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 52.3 | Aromatic Rings: | 1 |
| Heavy Atoms: | 11 | QED Weighted: | 0.488 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.481 | MDCK Permeability: | 0.00005090 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.947 |
| Human Intestinal Absorption (HIA): | 0.004 | 20% Bioavailability (F20%): | 0.005 |
| 30% Bioavailability (F30%): | 0.953 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.988 | Plasma Protein Binding (PPB): | 57.58% |
| Volume Distribution (VD): | 1.02 | Fu: | 44.46% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.948 | CYP1A2-substrate: | 0.749 |
| CYP2C19-inhibitor: | 0.675 | CYP2C19-substrate: | 0.683 |
| CYP2C9-inhibitor: | 0.253 | CYP2C9-substrate: | 0.566 |
| CYP2D6-inhibitor: | 0.064 | CYP2D6-substrate: | 0.745 |
| CYP3A4-inhibitor: | 0.135 | CYP3A4-substrate: | 0.219 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 8.392 | Half-life (T1/2): | 0.704 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.056 | Human Hepatotoxicity (H-HT): | 0.074 |
| Drug-inuced Liver Injury (DILI): | 0.32 | AMES Toxicity: | 0.063 |
| Rat Oral Acute Toxicity: | 0.034 | Maximum Recommended Daily Dose: | 0.016 |
| Skin Sensitization: | 0.42 | Carcinogencity: | 0.096 |
| Eye Corrosion: | 0.079 | Eye Irritation: | 0.991 |
| Respiratory Toxicity: | 0.844 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000160 |  |
0.703 | D07HBX | 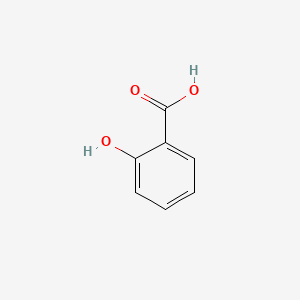 |
0.425 | ||
| ENC000104 |  |
0.667 | D0GY5Z | 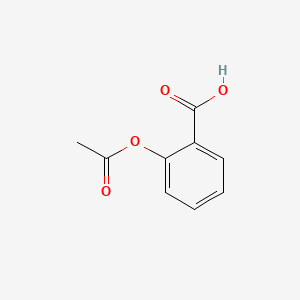 |
0.413 | ||
| ENC001356 |  |
0.545 | D0N3UL | 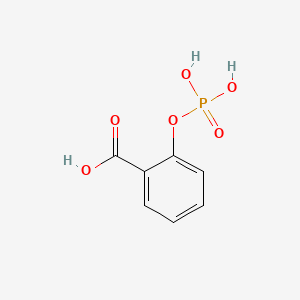 |
0.367 | ||
| ENC000299 |  |
0.545 | D0L5PO |  |
0.345 | ||
| ENC000174 |  |
0.526 | D0X9RY | 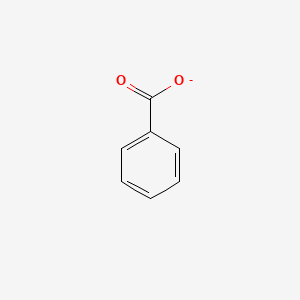 |
0.341 | ||
| ENC001345 |  |
0.523 | D0Q8ZX |  |
0.340 | ||
| ENC001333 |  |
0.463 | D0FN7J |  |
0.328 | ||
| ENC000108 |  |
0.462 | D0G2MH |  |
0.327 | ||
| ENC000846 |  |
0.441 | D0R1CR |  |
0.313 | ||
| ENC000302 |  |
0.439 | D0F5ZM |  |
0.309 | ||