NPs Basic Information

|
Name |
2-Acetylthiazole
|
| Molecular Formula | C5H5NOS | |
| IUPAC Name* |
1-(1,3-thiazol-2-yl)ethanone
|
|
| SMILES |
CC(=O)C1=NC=CS1
|
|
| InChI |
InChI=1S/C5H5NOS/c1-4(7)5-6-2-3-8-5/h2-3H,1H3
|
|
| InChIKey |
MOMFXATYAINJML-UHFFFAOYSA-N
|
|
| Synonyms |
2-Acetylthiazole; 24295-03-2; 1-(1,3-Thiazol-2-yl)ethan-1-one; 1-(1,3-Thiazol-2-yl)ethanone; Ethanone, 1-(2-thiazolyl)-; 1-thiazol-2-yl-ethanone; 2-ACETYL THIAZOLE; 1-(Thiazol-2-yl)ethan-1-one; Methyl 2-thiazolyl ketone; Ketone, methyl 2-thiazolyl; 1-(2-Thiazolyl)ethanone; 2-Thiazolyl methyl ketone; 1-(thiazol-2-yl)ethanone; Thiazole, 2-acetyl; 2-acetylthiazol; 2-acetyl-1,3-thiazole; FEMA No. 3328; MFCD00005324; 16IGS5268I; DSSTox_CID_10162; DSSTox_RID_78838; DSSTox_GSID_30162; acetylthiazole; CAS-24295-03-2; Methyl 5-thiazolyl ketone; 2acetylthiazole; UNII-16IGS5268I; 2 Acetylthiazole; 2-acetyl; 5-acetyl thiazole; thiazole-2-acetyl; 2-acetyl-; EINECS 246-134-5; 1-Thiazol-2-ylmethanone; 2-Acetylthiazole, 99%; 1-(2-thiazolyl)-ethanone; MLS002415692; SCHEMBL247631; 2-ACETYLTHIAZOLE [FHFI]; CHEMBL1589555; DTXSID0030162; 1-(2-Thiazolyl)ethanone, 9CI; FEMA 3328; Methyl 2-thiazolyl ketone, 8CI; 2-ACETYL THIAZOLE [FCC]; 2-Acetylthiazole, >=99%, FG; CHEBI:173474; HMS3039C05; ZINC164484; BCP27129; STR03999; Tox21_202054; Tox21_303515; AKOS005259005; AC-3209; CS-W008970; PB42359; PS-5288; NCGC00091718-01; NCGC00091718-02; NCGC00257353-01; NCGC00259603-01; 2-Thiazolyl methyl ketone2-Acetylthiazole; SMR001370882; SY004958; DB-020350; A1265; AM20090269; FT-0610984; EN300-67228; 95A032; F11223; Q-100310; Q27251815; F0001-0826; Z1069877574; 2 inverted exclamation mark -Chloro-biphenyl-4-carbaldehyde
|
|
| CAS | 24295-03-2 | |
| PubChem CID | 520108 | |
| ChEMBL ID | CHEMBL1589555 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 127.17 | ALogp: | 1.0 |
| HBD: | 0 | HBA: | 3 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 58.2 | Aromatic Rings: | 1 |
| Heavy Atoms: | 8 | QED Weighted: | 0.539 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.031 | MDCK Permeability: | 0.00009260 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0 |
| Human Intestinal Absorption (HIA): | 0.011 | 20% Bioavailability (F20%): | 0.019 |
| 30% Bioavailability (F30%): | 0.216 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.85 | Plasma Protein Binding (PPB): | 58.12% |
| Volume Distribution (VD): | 0.904 | Fu: | 63.17% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.965 | CYP1A2-substrate: | 0.89 |
| CYP2C19-inhibitor: | 0.664 | CYP2C19-substrate: | 0.43 |
| CYP2C9-inhibitor: | 0.062 | CYP2C9-substrate: | 0.798 |
| CYP2D6-inhibitor: | 0.184 | CYP2D6-substrate: | 0.712 |
| CYP3A4-inhibitor: | 0.024 | CYP3A4-substrate: | 0.27 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 4.51 | Half-life (T1/2): | 0.524 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.015 | Human Hepatotoxicity (H-HT): | 0.389 |
| Drug-inuced Liver Injury (DILI): | 0.818 | AMES Toxicity: | 0.789 |
| Rat Oral Acute Toxicity: | 0.466 | Maximum Recommended Daily Dose: | 0.029 |
| Skin Sensitization: | 0.591 | Carcinogencity: | 0.085 |
| Eye Corrosion: | 0.965 | Eye Irritation: | 0.996 |
| Respiratory Toxicity: | 0.979 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000163 |  |
0.375 | D0XF8W |  |
0.237 | ||
| ENC000648 | 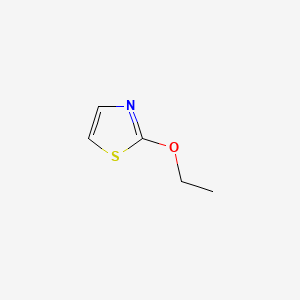 |
0.364 | D0S1NZ |  |
0.214 | ||
| ENC000650 | 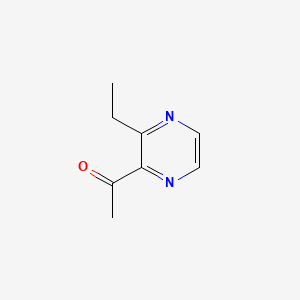 |
0.300 | D06NVJ | 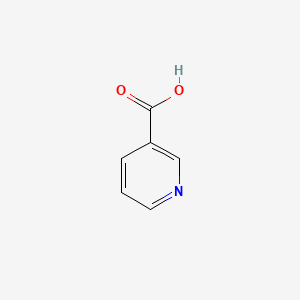 |
0.205 | ||
| ENC000480 |  |
0.257 | D04CRL | 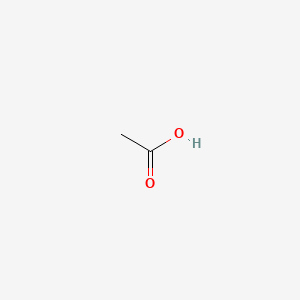 |
0.200 | ||
| ENC000640 |  |
0.243 | D0C1PY |  |
0.192 | ||
| ENC000192 |  |
0.237 | D0Z4UY |  |
0.192 | ||
| ENC000485 | 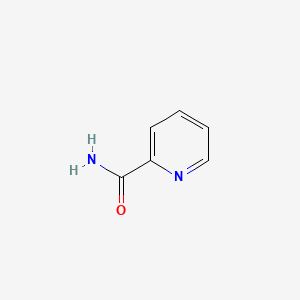 |
0.237 | D09XQF |  |
0.190 | ||
| ENC000056 | 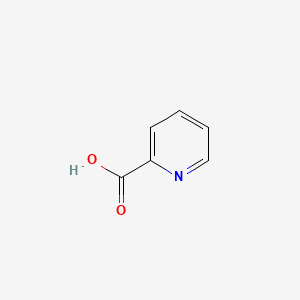 |
0.237 | D0I6IB |  |
0.186 | ||
| ENC000585 | 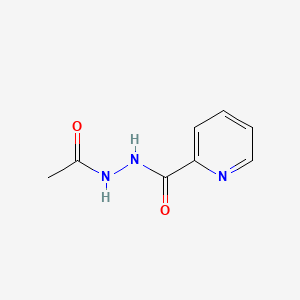 |
0.234 | D0U5QK |  |
0.182 | ||
| ENC000200 |  |
0.225 | D0P0HB |  |
0.182 | ||