NPs Basic Information

|
Name |
Clindamycin
|
| Molecular Formula | C18H33ClN2O5S | |
| IUPAC Name* |
(2S,4R)-N-[(1S,2S)-2-chloro-1-[(2R,3R,4S,5R,6R)-3,4,5-trihydroxy-6-methylsulfanyloxan-2-yl]propyl]-1-methyl-4-propylpyrrolidine-2-carboxamide
|
|
| SMILES |
CCC[C@@H]1C[C@H](N(C1)C)C(=O)N[C@@H]([C@@H]2[C@@H]([C@@H]([C@H]([C@H](O2)SC)O)O)O)[C@H](C)Cl
|
|
| InChI |
InChI=1S/C18H33ClN2O5S/c1-5-6-10-7-11(21(3)8-10)17(25)20-12(9(2)19)16-14(23)13(22)15(24)18(26-16)27-4/h9-16,18,22-24H,5-8H2,1-4H3,(H,20,25)/t9-,10+,11-,12+,13-,14+,15+,16+,18+/m0/s1
|
|
| InChIKey |
KDLRVYVGXIQJDK-AWPVFWJPSA-N
|
|
| Synonyms |
clindamycin; 18323-44-9; Chlolincocin; Clinimycin; Dalacine; Dalacin C; Sobelin; Clindamycin hydrochloride; Antirobe; 7(S)-Chloro-7-deoxylincomycin; 7-Chlorolincomycin; 7-Chloro-7-deoxylincomycin; U 21251; U-21251; U-21,251; Clindamycine; Klimicin; ClindaDerm; 3U02EL437C; Klindan 300; 7-Deoxy-7(S)-chlorolincomycin; Clindamycine [French]; Clindamycine [INN-French]; Clindamycinum [INN-Latin]; Clindamicina [INN-Spanish]; 7-CDL; (2S,4R)-N-[(1S,2S)-2-chloro-1-[(2R,3R,4S,5R,6R)-3,4,5-trihydroxy-6-methylsulfanyloxan-2-yl]propyl]-1-methyl-4-propylpyrrolidine-2-carboxamide; L-threo-a-D-galacto-Octopyranoside, methyl7-chloro-6,7,8-trideoxy-6-[[[(2S,4R)-1-methyl-4-propyl-2-pyrrolidinyl]carbonyl]amino]-1-thio-; methyl 7-chloro-6,7,8-trideoxy-6-{[(4R)-1-methyl-4-propyl-L-prolyl]amino}-1-thio-L-threo-alpha-D-galacto-octopyranoside; Clindamicina; Clindamycinum; (2S,4R)-N-((1S,2S)-2-chloro-1-((2R,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(methylthio)tetrahydro-2H-pyran-2-yl)propyl)-1-methyl-4-propylpyrrolidine-2-carboxamide; CLDM; UNII-3U02EL437C; Clindamycin [USAN:INN:BAN]; SR-05000001477; HSDB 3037; Clindamycin,(S); (2S,4R)-N-[(1S,2S)-2-chloro-1-[(2R,3R,4S,5R,6R)-3,4,5-trihydroxy-6-methylsulfanyl-tetrahydropyran-2-yl]propyl]-1-methyl-4-propyl-pyrrolidine-2-carboxamide; EINECS 242-209-1; NSC305832; CLINDAMYCIN [MI]; 58207-19-5; CLINDAMYCIN [INN]; Clindamycin (USAN/INN); CLINDAMYCIN [HSDB]; CLINDAMYCIN [USAN]; CLINDAMYCIN [VANDF]; SCHEMBL3154; CHEMBL1753; CLINDAMYCIN [MART.]; CLINDA & IL-12; CLINDAMYCIN [WHO-DD]; BIDD:GT0418; Clindamycin & Interleukin 12; CLINDAMYCIN [GREEN BOOK]; DTXSID2022836; GTPL10607; HMS2089A05; HMS2089N16; HY-B1455; L-THREO-.ALPHA.-D-GALACTO-OCTOPYRANOSIDE, METHYL 7-CHLORO-6,7,8-TRIDEOXY-6-(((1-METHYL-4-PROPYL-2-PYRROLIDINYL)CARBONYL)AMINO)-1-THIO-, (2S-TRANS)-; Methyl 7-chloro-6,7,8-trideoxy-6-(1-methyl-trans-4-propyl-L-2-pyrrolidinecarboxamido)-1-thio-L-threo-.alpha.-D-galacto-octopyranoside; ZINC4038341; AKOS037515852; AM84752; CCG-268941; CS-11147; L-threo-.alpha.-D-galacto-Octopyranoside, methyl 7-chloro-6,7,8-trideoxy-6-[[[(2S,4R)-1-methyl-4-propyl-2-pyrrolidinyl]carbonyl]amino]-1-thio-; L-threo-alpha-D-galacto-Octopyranoside, methyl 7-chloro-6,7,8-trideoxy-6-(((1-methyl-4-propyl-2-pyrrolidinyl)carbonyl)amino)-1-thio-, (2S-trans)-; Clindamycin 1000 microg/mL in Acetonitrile; CS-0013163; C06914; D00277; AB01275425-01; AB01275425_02; 323C449; A831786; EN300-19736038; SR-05000001477-1; CLINDAMYCIN PHOSPHATE IMPURITY E [EP IMPURITY]; (2S,4R)-N-[(1S,2S)-2-chloranyl-1-[(2R,3R,4S,5R,6R)-6-methylsulfanyl-3,4,5-tris(oxidanyl)oxan-2-yl]propyl]-1-methyl-4-propyl-pyrrolidine-2-carboxamide; (2S,4R)-N-[(1S,2S)-2-chloro-1-[(2R,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(methylsulfanyl)oxan-2-yl]propyl]-1-methyl-4-propylpyrrolidine-2-carboxamide; (2S,4R)-N-[(1S,2S)-2-chloro-1-[(2R,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(methylthio)-2-oxanyl]propyl]-1-methyl-4-propyl-2-pyrrolidinecarboxamide; L-threo-?-D-galacto-Octopyranoside, methyl 7-chloro-6,7,8-trideoxy-6-[[[(2S,4R)-1-methyl-4-propyl-2-pyrrolidinyl]carbonyl]amino]-1-thio-; L-threo-alpha-D-galacto-Octopyranoside, methyl 7-chloro-6,7,8-trideoxy-6-((((2S,4R)-1-methyl-4-propyl-2-pyrrolidinyl)carbonyl)amino)-1-thio-; L-threo-D-galacto-Octopyranoside, methyl 7-chloro-6,7,8-trideoxy-6-(1-methyl-4-propyl-L-2-pyrrolidinecarboxamido)-1-thio-, trans-alpha-; methyl 7-chloro-6,7,8-trideoxy-6-({[(2S,4R)-1-methyl-4-propyl-2-pyrrolidinyl]carbonyl}amino)-1-thio-L-threo-alpha-D-galacto-octopyranoside
|
|
| CAS | 18323-44-9 | |
| PubChem CID | 446598 | |
| ChEMBL ID | CHEMBL1753 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 425.0 | ALogp: | 2.2 |
| HBD: | 4 | HBA: | 7 |
| Rotatable Bonds: | 7 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 128.0 | Aromatic Rings: | 2 |
| Heavy Atoms: | 27 | QED Weighted: | 0.448 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.393 | MDCK Permeability: | 0.00001820 |
| Pgp-inhibitor: | 0.974 | Pgp-substrate: | 0.979 |
| Human Intestinal Absorption (HIA): | 0.857 | 20% Bioavailability (F20%): | 0.003 |
| 30% Bioavailability (F30%): | 0.009 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.082 | Plasma Protein Binding (PPB): | 90.99% |
| Volume Distribution (VD): | 0.767 | Fu: | 2.86% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.068 | CYP1A2-substrate: | 0.272 |
| CYP2C19-inhibitor: | 0.039 | CYP2C19-substrate: | 0.893 |
| CYP2C9-inhibitor: | 0.012 | CYP2C9-substrate: | 0.155 |
| CYP2D6-inhibitor: | 0.011 | CYP2D6-substrate: | 0.395 |
| CYP3A4-inhibitor: | 0.008 | CYP3A4-substrate: | 0.301 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 2.208 | Half-life (T1/2): | 0.862 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.046 | Human Hepatotoxicity (H-HT): | 0.902 |
| Drug-inuced Liver Injury (DILI): | 0.387 | AMES Toxicity: | 0.079 |
| Rat Oral Acute Toxicity: | 0.488 | Maximum Recommended Daily Dose: | 0.023 |
| Skin Sensitization: | 0.148 | Carcinogencity: | 0.111 |
| Eye Corrosion: | 0.003 | Eye Irritation: | 0.012 |
| Respiratory Toxicity: | 0.902 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC002431 |  |
0.272 | D0R0ZL |  |
1.000 | ||
| ENC005771 | 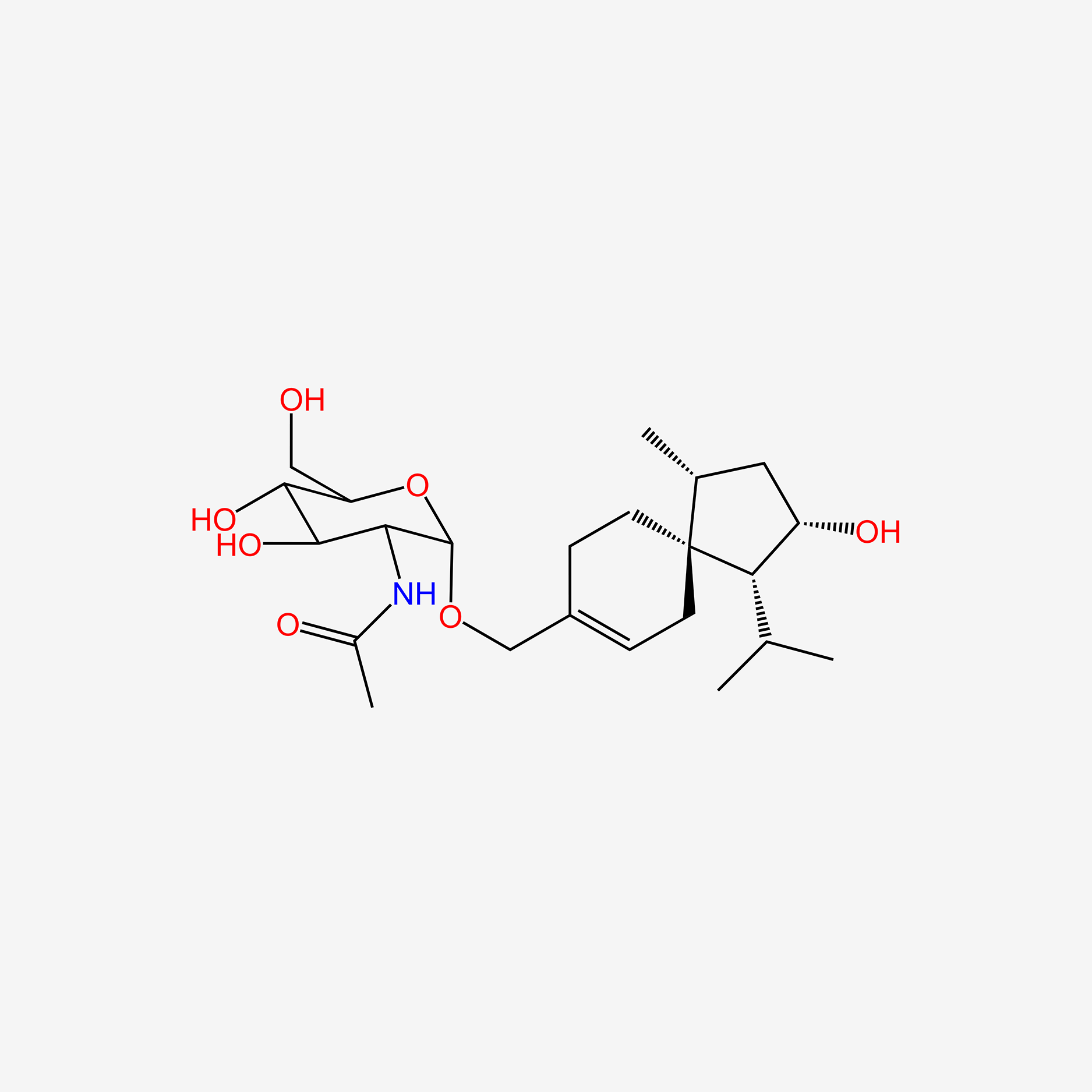 |
0.258 | D0Q0EX |  |
0.870 | ||
| ENC004407 |  |
0.252 | D05ZYM | 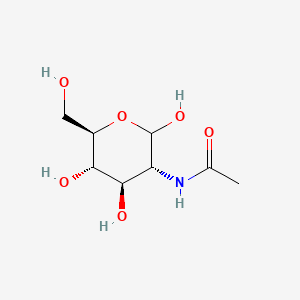 |
0.233 | ||
| ENC003055 | 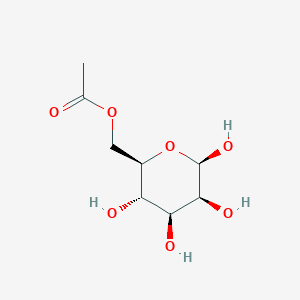 |
0.247 | D0P2IW |  |
0.222 | ||
| ENC001214 |  |
0.247 | D0M4WA |  |
0.222 | ||
| ENC001062 | 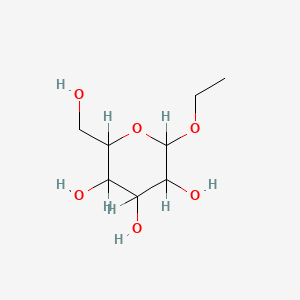 |
0.239 | D0HR8Z |  |
0.217 | ||
| ENC003068 | 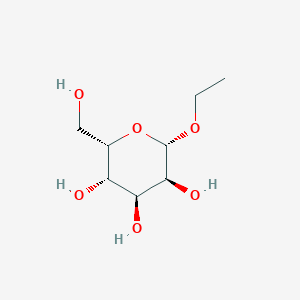 |
0.239 | D09MPU |  |
0.217 | ||
| ENC003707 |  |
0.238 | D0I8RR | 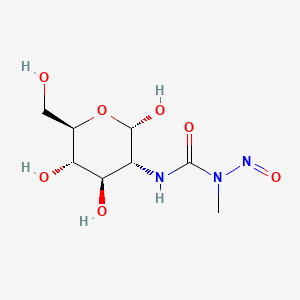 |
0.214 | ||
| ENC003582 | 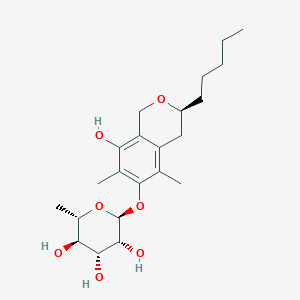 |
0.238 | D0PI3Z | 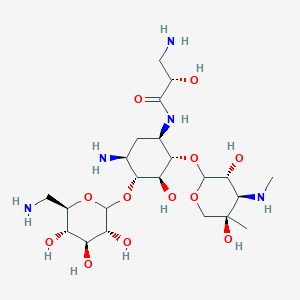 |
0.214 | ||
| ENC003177 | 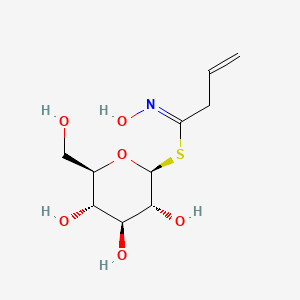 |
0.237 | D0H3KI | 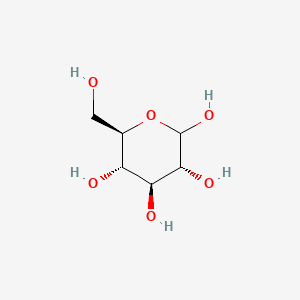 |
0.212 | ||