NPs Basic Information

|
Name |
diprotin A
|
| Molecular Formula | C17H31N3O4 | |
| IUPAC Name* |
(2S,3S)-2-[[(2S)-1-[(2S,3S)-2-amino-3-methylpentanoyl]pyrrolidine-2-carbonyl]amino]-3-methylpentanoic acid
|
|
| SMILES |
CC[C@H](C)[C@@H](C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)CC)C(=O)O)N
|
|
| InChI |
InChI=1S/C17H31N3O4/c1-5-10(3)13(18)16(22)20-9-7-8-12(20)15(21)19-14(17(23)24)11(4)6-2/h10-14H,5-9,18H2,1-4H3,(H,19,21)(H,23,24)/t10-,11-,12-,13-,14-/m0/s1
|
|
| InChIKey |
JNTMAZFVYNDPLB-PEDHHIEDSA-N
|
|
| Synonyms |
diprotin A; 90614-48-5; Ile-Pro-Ile; isoleucyl-prolyl-isoleucine; N-(1-L-Isoleucyl-L-prolyl)-L-isoleucine; l-isoleucyl-l-prolyl-l-isoleucine; CHEMBL214381; isoleucylprolylisoleucine; (2S,3S)-2-((S)-1-((2S,3S)-2-Amino-3-methylpentanoyl)pyrrolidine-2-carboxamido)-3-methylpentanoic acid; H-Ile-Pro-Ile-OH; MFCD00038707; Spectrum2_001480; Spectrum3_001838; L-Isoleucine, N-(1-L-isoleucyl-L-prolyl)-; Diprotin A (Ile-Pro-Ile); BSPBio_003515; SPBio_001439; SCHEMBL6404766; BCBcMAP01_000164; CHEBI:93213; KBio3_003020; Dipeptidyl peptidase IV inhibitor; DTXSID80920277; ZINC4899477; BDBM50229666; CCG-38872; AKOS030210980; SMP1_000085; NCGC00178015-01; Diprotin A (H-L-Ile-L-Pro-L-Ile-OH); HY-111174; CS-0034521; BRD-K69032158-001-02-2; Q27164930; N-[Hydroxy(1-isoleucylpyrrolidin-2-yl)methylidene]isoleucine; (2S,3S)-2-((S)-1-((2S,3S)-2-amino-3-methylpentanoyl)pyrrolidine-5-carboxamido)-3-methylpentanoic acid; (2S,3S)-2-[[[(2S)-1-[(2S,3S)-2-amino-3-methyl-1-oxopentyl]-2-pyrrolidinyl]-oxomethyl]amino]-3-methylpentanoic acid; IPI
|
|
| CAS | 90614-48-5 | |
| PubChem CID | 94701 | |
| ChEMBL ID | CHEMBL214381 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 341.4 | ALogp: | -1.0 |
| HBD: | 3 | HBA: | 5 |
| Rotatable Bonds: | 8 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 113.0 | Aromatic Rings: | 1 |
| Heavy Atoms: | 24 | QED Weighted: | 0.617 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.913 | MDCK Permeability: | 0.00010047 |
| Pgp-inhibitor: | 0.016 | Pgp-substrate: | 0.035 |
| Human Intestinal Absorption (HIA): | 0.376 | 20% Bioavailability (F20%): | 0.007 |
| 30% Bioavailability (F30%): | 0.006 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.898 | Plasma Protein Binding (PPB): | 32.13% |
| Volume Distribution (VD): | 0.391 | Fu: | 59.43% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.004 | CYP1A2-substrate: | 0.058 |
| CYP2C19-inhibitor: | 0.066 | CYP2C19-substrate: | 0.385 |
| CYP2C9-inhibitor: | 0.025 | CYP2C9-substrate: | 0.161 |
| CYP2D6-inhibitor: | 0.031 | CYP2D6-substrate: | 0.15 |
| CYP3A4-inhibitor: | 0.032 | CYP3A4-substrate: | 0.113 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 4.362 | Half-life (T1/2): | 0.879 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.008 | Human Hepatotoxicity (H-HT): | 0.555 |
| Drug-inuced Liver Injury (DILI): | 0.097 | AMES Toxicity: | 0.003 |
| Rat Oral Acute Toxicity: | 0.069 | Maximum Recommended Daily Dose: | 0.045 |
| Skin Sensitization: | 0.046 | Carcinogencity: | 0.022 |
| Eye Corrosion: | 0.003 | Eye Irritation: | 0.014 |
| Respiratory Toxicity: | 0.141 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001093 |  |
0.591 | D0X5SJ |  |
0.386 | ||
| ENC000749 | 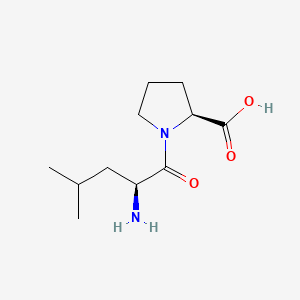 |
0.438 | D0I0EG | 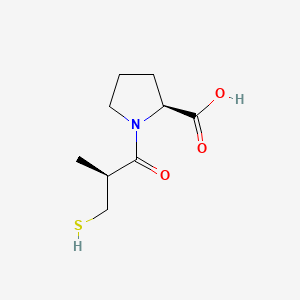 |
0.370 | ||
| ENC002115 |  |
0.369 | D0N5HJ | 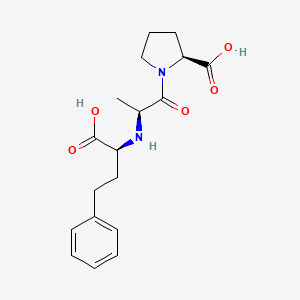 |
0.347 | ||
| ENC000918 | 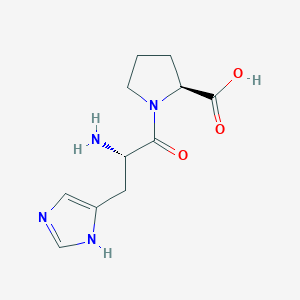 |
0.314 | D00SEB | 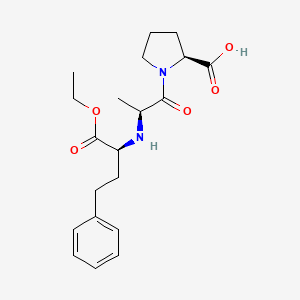 |
0.340 | ||
| ENC000141 |  |
0.308 | D03KYG | 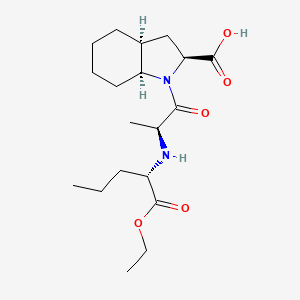 |
0.301 | ||
| ENC001906 |  |
0.303 | D07HGR | 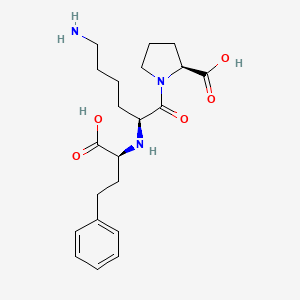 |
0.297 | ||
| ENC003576 | 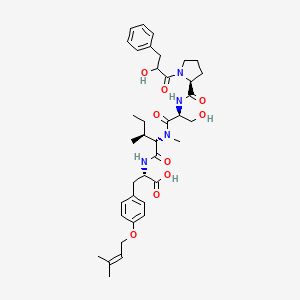 |
0.301 | D08BTB |  |
0.283 | ||
| ENC002451 | 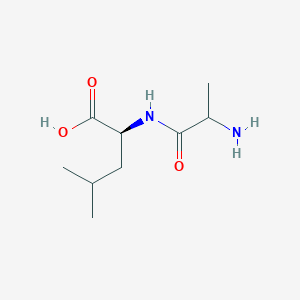 |
0.289 | D07WXE | 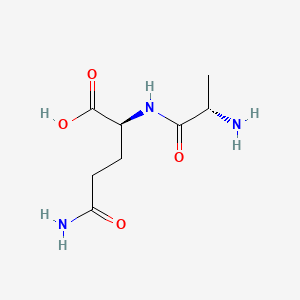 |
0.278 | ||
| ENC001171 | 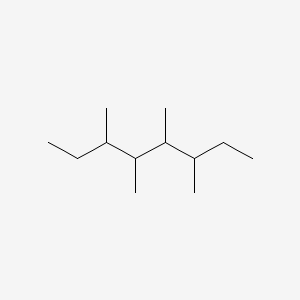 |
0.274 | D0N4EC | 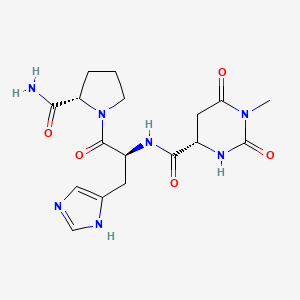 |
0.277 | ||
| ENC001514 |  |
0.273 | D0P2IW |  |
0.255 | ||