NPs Basic Information
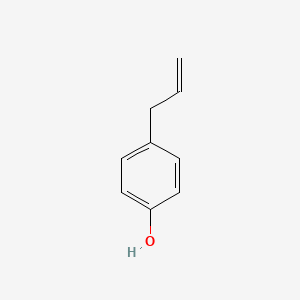
|
Name |
4-Allylphenol
|
| Molecular Formula | C9H10O | |
| IUPAC Name* |
4-prop-2-enylphenol
|
|
| SMILES |
C=CCC1=CC=C(C=C1)O
|
|
| InChI |
InChI=1S/C9H10O/c1-2-3-8-4-6-9(10)7-5-8/h2,4-7,10H,1,3H2
|
|
| InChIKey |
RGIBXDHONMXTLI-UHFFFAOYSA-N
|
|
| Synonyms |
4-Allylphenol; Chavicol; 501-92-8; p-Allylphenol; p-Hydroxyallylbenzene; Phenol, 4-(2-propenyl)-; 4-prop-2-enylphenol; Phenol, p-allyl-; 4-(prop-2-en-1-yl)phenol; p-Chavicol; 4-(2-Propenyl)phenol; gamma-(p-Hydroxyphenyl)-alpha-propylene; 4-(Prop-2-enyl)-phenol; 4-Allyl-Phenol; 3-(4-Hydroxyphenyl)-1-propene; .gamma.-(p-Hydroxyphenyl)-.alpha.-propylene; CHEBI:50158; Q5ER4K6969; MFCD01940501; NSC-290195; CCRIS 3208; EINECS 207-929-2; NSC 290195; UNII-Q5ER4K6969; alpha -propylene; p-Allyl-Phenol; p-Hydroxyallylpropene; CHAVICOL [MI]; 4-(2-propenyl)-phenol; Phenol, p-allyl- (8CI); SCHEMBL30870; 4-ALLYLPHENOL [FHFI]; CHEMBL108862; 3-(p-Hydroxyphenyl)-1-propene; FEMA NO. 4075; RGIBXDHONMXTLI-UHFFFAOYSA-; DTXSID60198210; ZINC1565456; AC1877; NSC290195; Phenol, 4-(2-propenyl)- (9CI); AKOS006278514; CS-12298; SY045829; DB-106539; C3743; CS-0217169; C16930; EN300-698378; 501A928; A917800; laquo gammaRaquo -(p-hydroxyphenyl)-alpha -propylene; Q2504388
|
|
| CAS | 501-92-8 | |
| PubChem CID | 68148 | |
| ChEMBL ID | CHEMBL108862 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 134.17 | ALogp: | 2.9 |
| HBD: | 1 | HBA: | 1 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 20.2 | Aromatic Rings: | 1 |
| Heavy Atoms: | 10 | QED Weighted: | 0.616 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.377 | MDCK Permeability: | 0.00002970 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.884 |
| 30% Bioavailability (F30%): | 0.91 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.126 | Plasma Protein Binding (PPB): | 89.82% |
| Volume Distribution (VD): | 0.828 | Fu: | 6.39% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.899 | CYP1A2-substrate: | 0.586 |
| CYP2C19-inhibitor: | 0.783 | CYP2C19-substrate: | 0.365 |
| CYP2C9-inhibitor: | 0.265 | CYP2C9-substrate: | 0.877 |
| CYP2D6-inhibitor: | 0.771 | CYP2D6-substrate: | 0.9 |
| CYP3A4-inhibitor: | 0.134 | CYP3A4-substrate: | 0.308 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 15.156 | Half-life (T1/2): | 0.889 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.017 | Human Hepatotoxicity (H-HT): | 0.031 |
| Drug-inuced Liver Injury (DILI): | 0.029 | AMES Toxicity: | 0.076 |
| Rat Oral Acute Toxicity: | 0.219 | Maximum Recommended Daily Dose: | 0.096 |
| Skin Sensitization: | 0.888 | Carcinogencity: | 0.569 |
| Eye Corrosion: | 0.918 | Eye Irritation: | 0.99 |
| Respiratory Toxicity: | 0.189 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000310 | 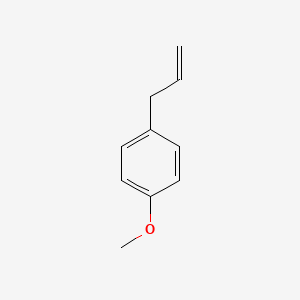 |
0.595 | D0W1RY |  |
0.513 | ||
| ENC000350 |  |
0.556 | D03UOT |  |
0.471 | ||
| ENC000740 |  |
0.556 | D01CRB |  |
0.465 | ||
| ENC000006 |  |
0.526 | D0B3QM |  |
0.444 | ||
| ENC000774 | 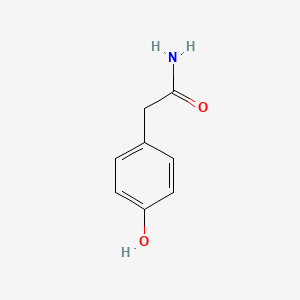 |
0.526 | D0S2BV |  |
0.400 | ||
| ENC004860 |  |
0.488 | D0U5QK |  |
0.381 | ||
| ENC000005 |  |
0.472 | D0H6TP |  |
0.333 | ||
| ENC000086 |  |
0.471 | D02WAB |  |
0.327 | ||
| ENC001021 |  |
0.471 | D0K1QD | 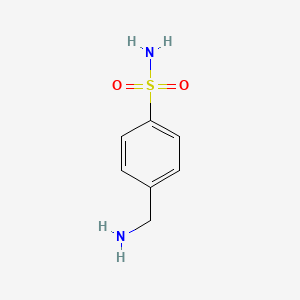 |
0.304 | ||
| ENC000129 |  |
0.465 | D00LFB |  |
0.299 | ||