NPs Basic Information

|
Name |
6,6-Dimethylbicyclo[3.1.1]heptan-2-one
|
| Molecular Formula | C9H14O | |
| IUPAC Name* |
6,6-dimethylbicyclo[3.1.1]heptan-2-one
|
|
| SMILES |
CC1(C2CCC(=O)C1C2)C
|
|
| InChI |
InChI=1S/C9H14O/c1-9(2)6-3-4-8(10)7(9)5-6/h6-7H,3-5H2,1-2H3
|
|
| InChIKey |
XZFDKWMYCUEKSS-UHFFFAOYSA-N
|
|
| Synonyms |
6,6-Dimethylbicyclo[3.1.1]heptan-2-one; Nopinone; 24903-95-5; Nopinon; beta-Pinone; .beta.-Pinone; Bicyclo[3.1.1]heptan-2-one, 6,6-dimethyl-; 2-Norpinanone, 6,6-dimethyl-; 6,6-Dimethylbicyclo(3.1.1)heptan-2-one; Bicyclo(3.1.1)heptan-2-one, 6,6-dimethyl-; MFCD08447116; NSC-250997; EINECS 246-520-3; 6,6-dimethyl-bicyclo[3.1.1]heptan-2-one; NSC 135004; (1R)-(+)-Norinone; Bicyclo[3.1.1]heptan-2-one, 6,6-dimethyl-, (1R)-; 2-Norpinanone,6-dimethyl-; SCHEMBL720591; DTXSID20865173; CHEBI:181453; NSC135004; NSC250997; AKOS004907716; NSC-135004; SB45077; LS-13665; SY211609; Bicyclo[3.1.1]heptan-2-one,6-dimethyl-; DB-013534; CS-0101275; FT-0639485; EN300-182727; A936770; (1R)-6,6-DIMETHYLDICYCLO[3.1.1]HEPTAN-2-ONE
|
|
| CAS | 24903-95-5 | |
| PubChem CID | 32735 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 138.21 | ALogp: | 1.6 |
| HBD: | 0 | HBA: | 1 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 17.1 | Aromatic Rings: | 3 |
| Heavy Atoms: | 10 | QED Weighted: | 0.503 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.483 | MDCK Permeability: | 0.00002540 |
| Pgp-inhibitor: | 0.002 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.004 | 20% Bioavailability (F20%): | 0.004 |
| 30% Bioavailability (F30%): | 0.011 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.972 | Plasma Protein Binding (PPB): | 46.78% |
| Volume Distribution (VD): | 0.882 | Fu: | 49.78% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.095 | CYP1A2-substrate: | 0.343 |
| CYP2C19-inhibitor: | 0.07 | CYP2C19-substrate: | 0.782 |
| CYP2C9-inhibitor: | 0.215 | CYP2C9-substrate: | 0.873 |
| CYP2D6-inhibitor: | 0.003 | CYP2D6-substrate: | 0.825 |
| CYP3A4-inhibitor: | 0.015 | CYP3A4-substrate: | 0.226 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 13.867 | Half-life (T1/2): | 0.471 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.007 | Human Hepatotoxicity (H-HT): | 0.168 |
| Drug-inuced Liver Injury (DILI): | 0.125 | AMES Toxicity: | 0.008 |
| Rat Oral Acute Toxicity: | 0.092 | Maximum Recommended Daily Dose: | 0.419 |
| Skin Sensitization: | 0.128 | Carcinogencity: | 0.047 |
| Eye Corrosion: | 0.912 | Eye Irritation: | 0.936 |
| Respiratory Toxicity: | 0.531 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000482 |  |
0.636 | D0H1QY | 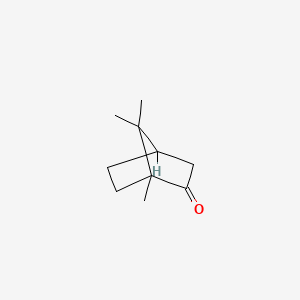 |
0.333 | ||
| ENC000153 |  |
0.421 | D0V8HA | 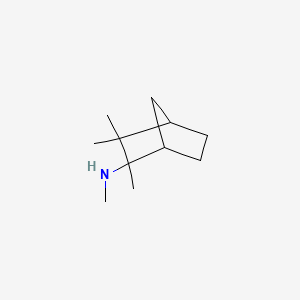 |
0.283 | ||
| ENC002084 | 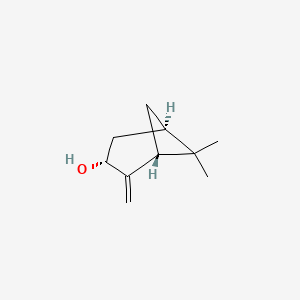 |
0.400 | D04DJN |  |
0.229 | ||
| ENC001814 |  |
0.366 | D0K0EK | 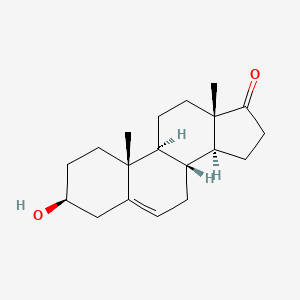 |
0.211 | ||
| ENC005520 |  |
0.366 | D06XMU |  |
0.211 | ||
| ENC002228 | 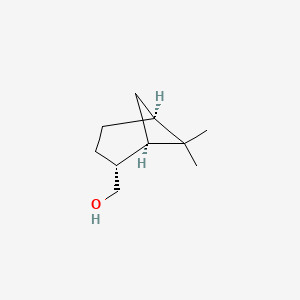 |
0.357 | D0U3GL | 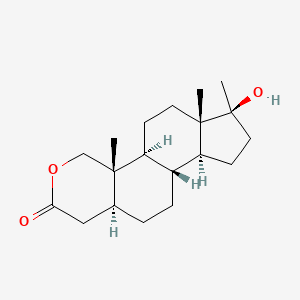 |
0.205 | ||
| ENC000151 |  |
0.350 | D0Z1XD | 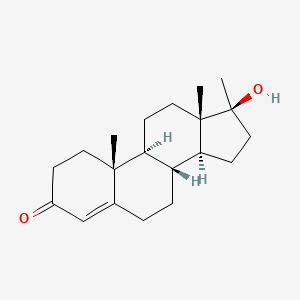 |
0.205 | ||
| ENC001898 | 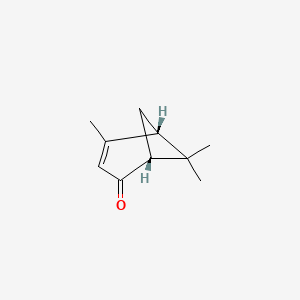 |
0.333 | D0D2VS |  |
0.205 | ||
| ENC000481 |  |
0.333 | D0I5DS | 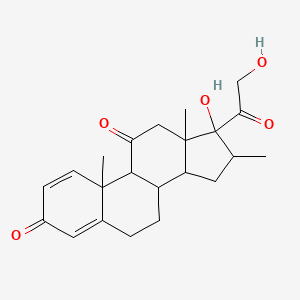 |
0.205 | ||
| ENC000830 |  |
0.333 | D0A2AJ |  |
0.203 | ||