NPs Basic Information

|
Name |
Fenchone
|
| Molecular Formula | C10H16O | |
| IUPAC Name* |
1,3,3-trimethylbicyclo[2.2.1]heptan-2-one
|
|
| SMILES |
CC1(C2CCC(C2)(C1=O)C)C
|
|
| InChI |
InChI=1S/C10H16O/c1-9(2)7-4-5-10(3,6-7)8(9)11/h7H,4-6H2,1-3H3
|
|
| InChIKey |
LHXDLQBQYFFVNW-UHFFFAOYSA-N
|
|
| Synonyms |
(.+/-.)-Fenchone; 1,3,3-Trimethylbicyclo[2.2.1]heptan-2-one; 1195-79-5; 214-804-6; CAS-1195-79-5; dl-fenchone; EINECS 214-804-6; Fenchone; fenchone, (+-)-isomer; alpha-Fenchone; 2-Norbornanone, 1,3,3-trimethyl-; Fenchon; 1,3,3-Trimethyl-2-norbornanone; 1,3,3-Trimethyl-2-norcamphanone; CHEBI:4999; MFCD00248429; Bicyclo[2.2.1]heptan-2-one, 1,3,3-trimethyl-; NCGC00166292-01; 1,3-Trimethylnorcamphor; NSC 8896; Bicyclo[2.2.1]heptan-2-one, 1,3,3-trimethyl-, (1S,4R)-; NSC 122687; 1,3-Trimethyl-2-norbornanone; 1,3-Trimethyl-2-norcamphanone; 2-Norbornanone,3,3-trimethyl-; WLN: L55 A CVTJ B1 D1 D1; 1,3-Trimethylbicyclo[2.2.1]heptan-2-one; Bicyclo[2.2.1]heptan-2-one,3,3-trimethyl-; NSC-8896; iso-Fenchone; fenchan-2-one; NSC-122687; DSSTox_CID_5324; DSSTox_RID_77748; DSSTox_GSID_25324; SCHEMBL57584; 126-21-6; CHEMBL2268554; DTXSID9025324; NSC8896; AMY25685; Tox21_112396; BBL018824; NSC122687; s5934; STK802502; AKOS009158536; LMPR0102120016; VS-06784; DB-017638; DB-070766; F0163; F0164; FT-0604551; FT-0627597; 1.3.3-trimethylbicyclo(2.2.1)heptan-2-one; EN300-54085; C09859; 1,3,3-Trimethyl-bicyclo[2.2.1]heptan-2-one; Bicyclo[2.2.1]heptane-2-one,1,3,3-trimethyl; A872224; Q414784; SR-01000945193; SR-01000945193-1; Z804948554; Bicyclo[2.2.1]heptan-2-one, 1,3,3-trimethyl-, (.+/-.)-
|
|
| CAS | 1195-79-5 | |
| PubChem CID | 14525 | |
| ChEMBL ID | CHEMBL2268554 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 152.23 | ALogp: | 2.3 |
| HBD: | 0 | HBA: | 1 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 17.1 | Aromatic Rings: | 2 |
| Heavy Atoms: | 11 | QED Weighted: | 0.521 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.618 | MDCK Permeability: | 0.00002220 |
| Pgp-inhibitor: | 0.012 | Pgp-substrate: | 0 |
| Human Intestinal Absorption (HIA): | 0.006 | 20% Bioavailability (F20%): | 0.011 |
| 30% Bioavailability (F30%): | 0.01 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.654 | Plasma Protein Binding (PPB): | 76.53% |
| Volume Distribution (VD): | 0.938 | Fu: | 40.05% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.042 | CYP1A2-substrate: | 0.906 |
| CYP2C19-inhibitor: | 0.447 | CYP2C19-substrate: | 0.927 |
| CYP2C9-inhibitor: | 0.082 | CYP2C9-substrate: | 0.767 |
| CYP2D6-inhibitor: | 0.01 | CYP2D6-substrate: | 0.551 |
| CYP3A4-inhibitor: | 0.083 | CYP3A4-substrate: | 0.378 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 11.416 | Half-life (T1/2): | 0.66 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.004 | Human Hepatotoxicity (H-HT): | 0.26 |
| Drug-inuced Liver Injury (DILI): | 0.095 | AMES Toxicity: | 0.018 |
| Rat Oral Acute Toxicity: | 0.13 | Maximum Recommended Daily Dose: | 0.106 |
| Skin Sensitization: | 0.058 | Carcinogencity: | 0.348 |
| Eye Corrosion: | 0.896 | Eye Irritation: | 0.932 |
| Respiratory Toxicity: | 0.973 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC002112 |  |
0.400 | D0H1QY |  |
0.487 | ||
| ENC001814 |  |
0.381 | D0V8HA |  |
0.298 | ||
| ENC005519 |  |
0.372 | D0U3GL |  |
0.250 | ||
| ENC000085 | 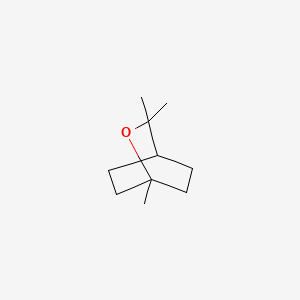 |
0.372 | D0L2LS | 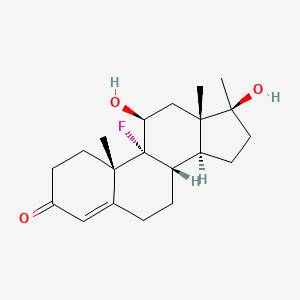 |
0.237 | ||
| ENC001047 | 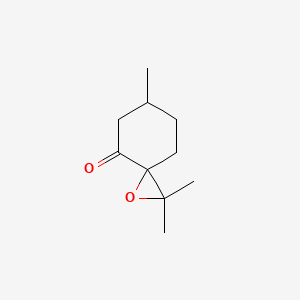 |
0.364 | D0Q6NZ | 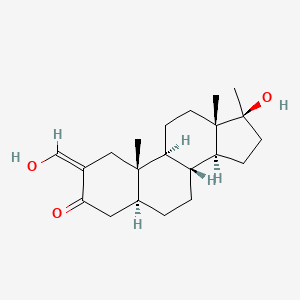 |
0.234 | ||
| ENC002662 |  |
0.347 | D0Z1XD | 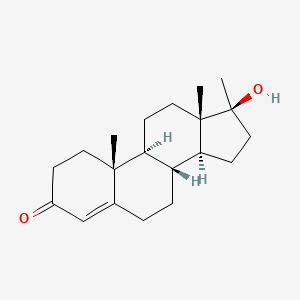 |
0.233 | ||
| ENC000613 |  |
0.333 | D04DJN |  |
0.222 | ||
| ENC002337 |  |
0.327 | D07QKN | 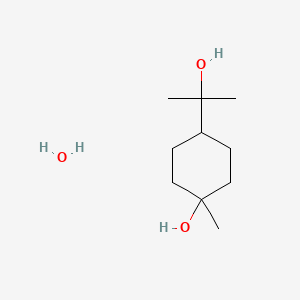 |
0.220 | ||
| ENC002074 |  |
0.327 | D09NNA |  |
0.217 | ||
| ENC002301 | 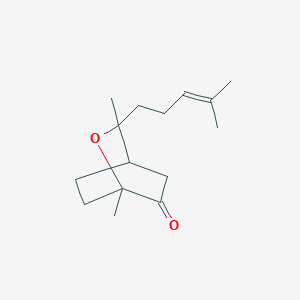 |
0.316 | D0A2AJ |  |
0.215 | ||