NPs Basic Information
|
Name |
Dioctyl adipate
|
| Molecular Formula | C22H42O4 | |
| IUPAC Name* |
dioctyl hexanedioate
|
|
| SMILES |
CCCCCCCCOC(=O)CCCCC(=O)OCCCCCCCC
|
|
| InChI |
InChI=1S/C22H42O4/c1-3-5-7-9-11-15-19-25-21(23)17-13-14-18-22(24)26-20-16-12-10-8-6-4-2/h3-20H2,1-2H3
|
|
| InChIKey |
NEHDRDVHPTWWFG-UHFFFAOYSA-N
|
|
| Synonyms |
Dioctyl adipate; Dioctyl hexanedioate; 123-79-5; Di-n-octyl adipate; Hexanedioic acid, dioctyl ester; Dicaprylyl adipate; Adipic acid, dioctyl ester; Hexanedioic acid, 1,6-dioctyl ester; 1,6-dioctyl hexanedioate; NSC 16201; 2BD76YG9SI; NSC-16201; HSDB 366; EINECS 204-652-9; UNII-2BD76YG9SI; AI3-17824; Adiimoll DO; Adipic acid dioctyl; SCHEMBL15509; Dioctyl ester hexanedioic acid; Hexanedioic acid dioctyl ester; Adipic acid, bis-n-octyl ester; DTXSID2021606; FEMA NO. 4476; Adipic acid di-n-octyl ester 5g; DIOCTYL ADIPATE [WHO-DD]; Dioctyl ester of hexanedioic acid; CHEBI:168986; DI-N-OCTYL ADIPATE [HSDB]; NSC16201; BBL010773; LMFA07010840; MFCD00072258; STK584146; ZINC59301183; AKOS005507485; AS-62479; FT-0621921; H10660; J-004984; J-520375; Q4731704
|
|
| CAS | 123-79-5 | |
| PubChem CID | 31271 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 370.6 | ALogp: | 7.4 |
| HBD: | 0 | HBA: | 4 |
| Rotatable Bonds: | 21 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 52.6 | Aromatic Rings: | 0 |
| Heavy Atoms: | 26 | QED Weighted: | 0.197 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.813 | MDCK Permeability: | 0.00001930 |
| Pgp-inhibitor: | 0.113 | Pgp-substrate: | 0.008 |
| Human Intestinal Absorption (HIA): | 0.002 | 20% Bioavailability (F20%): | 0.997 |
| 30% Bioavailability (F30%): | 0.999 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.081 | Plasma Protein Binding (PPB): | 96.40% |
| Volume Distribution (VD): | 0.962 | Fu: | 1.64% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.617 | CYP1A2-substrate: | 0.169 |
| CYP2C19-inhibitor: | 0.483 | CYP2C19-substrate: | 0.057 |
| CYP2C9-inhibitor: | 0.321 | CYP2C9-substrate: | 0.842 |
| CYP2D6-inhibitor: | 0.23 | CYP2D6-substrate: | 0.035 |
| CYP3A4-inhibitor: | 0.57 | CYP3A4-substrate: | 0.078 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 8.835 | Half-life (T1/2): | 0.413 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.431 | Human Hepatotoxicity (H-HT): | 0.012 |
| Drug-inuced Liver Injury (DILI): | 0.184 | AMES Toxicity: | 0.003 |
| Rat Oral Acute Toxicity: | 0.021 | Maximum Recommended Daily Dose: | 0.015 |
| Skin Sensitization: | 0.965 | Carcinogencity: | 0.122 |
| Eye Corrosion: | 0.962 | Eye Irritation: | 0.92 |
| Respiratory Toxicity: | 0.296 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001218 | 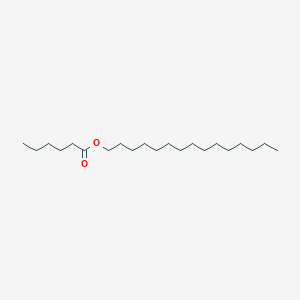 |
0.750 | D00MLW | 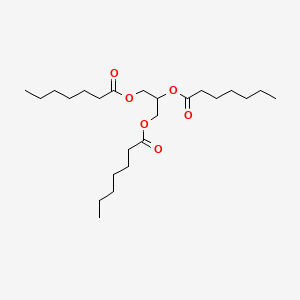 |
0.596 | ||
| ENC003079 | 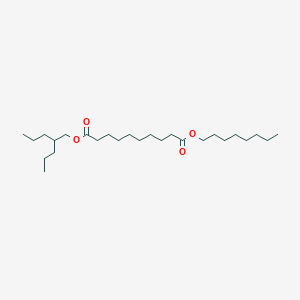 |
0.728 | D07ILQ |  |
0.489 | ||
| ENC001234 |  |
0.718 | D00FGR |  |
0.461 | ||
| ENC000765 |  |
0.674 | D0Z5SM | 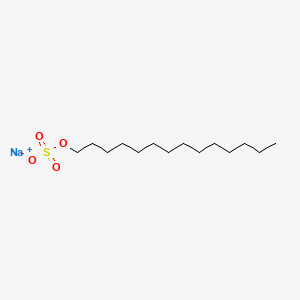 |
0.455 | ||
| ENC001202 | 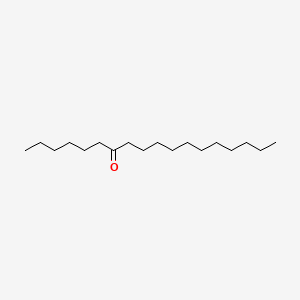 |
0.620 | D0O1PH |  |
0.429 | ||
| ENC001236 | 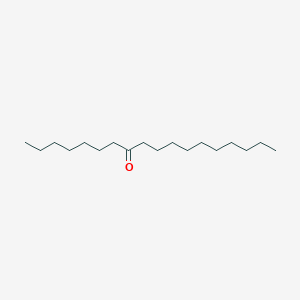 |
0.620 | D05ATI | 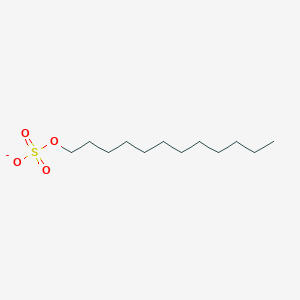 |
0.424 | ||
| ENC000419 |  |
0.617 | D00AOJ |  |
0.410 | ||
| ENC000575 |  |
0.614 | D0T9TJ |  |
0.398 | ||
| ENC000258 |  |
0.612 | D0AY9Q |  |
0.398 | ||
| ENC001243 |  |
0.609 | D0Z1QC |  |
0.371 | ||