NPs Basic Information
|
Name |
2,2,6,6-Tetramethyl-4-piperidone
|
| Molecular Formula | C9H17NO | |
| IUPAC Name* |
2,2,6,6-tetramethylpiperidin-4-one
|
|
| SMILES |
CC1(CC(=O)CC(N1)(C)C)C
|
|
| InChI |
InChI=1S/C9H17NO/c1-8(2)5-7(11)6-9(3,4)10-8/h10H,5-6H2,1-4H3
|
|
| InChIKey |
JWUXJYZVKZKLTJ-UHFFFAOYSA-N
|
|
| Synonyms |
Triacetonamine; 826-36-8; 2,2,6,6-Tetramethyl-4-piperidone; 2,2,6,6-tetramethylpiperidin-4-one; Vincubine; Triacetone amine; Triacetonamin; 2,2,6,6-Tetramethyl-4-piperidinone; Vincubina; Tempidon; 2,2,6,6-Tetramethyl-4-oxopiperidine; 4-Piperidinone, 2,2,6,6-tetramethyl-; Odoratine; Trojacetonoaminy; 2,2,6,6-Tetramethylpiperidone; 4-Oxo-2,2,6,6-tetramethylpiperidine; TMPone; 2,2,6,6-Tetramethylpiperidinone; Triacetoneamine; 2,2,6,6-Tetramethyl-piperidin-4-one; NSC 16579; 4-Oxo-2,2,6,6-tetramethyl-4-piperidone; 4-PIPERIDONE, 2,2,6,6-TETRAMETHYL-; 2K4430S3XP; NSC-16579; Trojacetonoaminy [Polish]; Tetramethylpiperidinone; EINECS 212-554-2; BRN 0112665; UNII-2K4430S3XP; Odoratin?; EC 212-554-2; Ikh 196; DSSTox_CID_21527; DSSTox_RID_79766; DSSTox_GSID_41527; Oprea1_386573; SCHEMBL38953; 5-21-06-00538 (Beilstein Handbook Reference); 2,2,6,6-tetramethylpiperidone-4-toluene-p- sulfonate; CHEMBL117614; IKH-19; DTXSID4041527; JWUXJYZVKZKLTJ-UHFFFAOYSA-; 2,6,6-Tetramethyl-4-piperidone; CHEBI:177813; 2,6,6-Tetramethyl-4-piperidinone; 2,2,6,6-teramethyl-4-piperidone; HY-N1131; NSC16579; STR06804; ZINC1747064; 2,2,6,6-Tetramethyl-g-piperidone; 2,6,6-Tetramethyl-4-oxopiperidine; Tox21_300816; CCG-44306; MFCD00005975; STK256617; AKOS000120903; AC-2702; SB74533; 2,2,6, 6-Tetramethyl-4-piperidinone; NCGC00248182-01; NCGC00248182-02; NCGC00254720-01; CAS-826-36-8; 2,2,6,6-Tetramethyl-4-piperidone, 95%; TO0127900; CS-0016419; FT-0609128; S4859; T1424; EN300-18105; 26T368; P19976; AB00375601-03; Q6120732; SR-01000634150-1; W-104169; F8889-5387; Z1250100692
|
|
| CAS | 826-36-8 | |
| PubChem CID | 13220 | |
| ChEMBL ID | CHEMBL117614 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 155.24 | ALogp: | 0.5 |
| HBD: | 1 | HBA: | 2 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 29.1 | Aromatic Rings: | 1 |
| Heavy Atoms: | 11 | QED Weighted: | 0.58 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.608 | MDCK Permeability: | 0.00002350 |
| Pgp-inhibitor: | 0.005 | Pgp-substrate: | 0.002 |
| Human Intestinal Absorption (HIA): | 0.012 | 20% Bioavailability (F20%): | 0.131 |
| 30% Bioavailability (F30%): | 0.07 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.945 | Plasma Protein Binding (PPB): | 23.41% |
| Volume Distribution (VD): | 1.141 | Fu: | 81.50% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.011 | CYP1A2-substrate: | 0.183 |
| CYP2C19-inhibitor: | 0.02 | CYP2C19-substrate: | 0.924 |
| CYP2C9-inhibitor: | 0.001 | CYP2C9-substrate: | 0.449 |
| CYP2D6-inhibitor: | 0.49 | CYP2D6-substrate: | 0.901 |
| CYP3A4-inhibitor: | 0.004 | CYP3A4-substrate: | 0.476 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 6.101 | Half-life (T1/2): | 0.808 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.005 | Human Hepatotoxicity (H-HT): | 0.175 |
| Drug-inuced Liver Injury (DILI): | 0.028 | AMES Toxicity: | 0.011 |
| Rat Oral Acute Toxicity: | 0.019 | Maximum Recommended Daily Dose: | 0.92 |
| Skin Sensitization: | 0.648 | Carcinogencity: | 0.129 |
| Eye Corrosion: | 0.316 | Eye Irritation: | 0.175 |
| Respiratory Toxicity: | 0.858 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
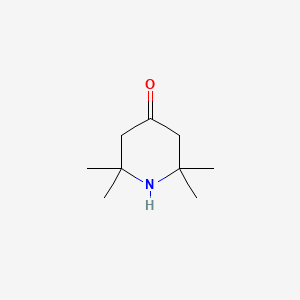
| ENC001332 | 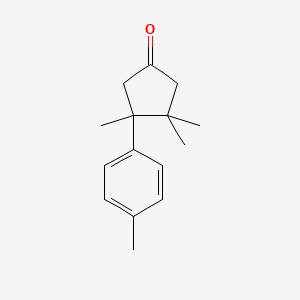 |
0.340 | D0H1QY | 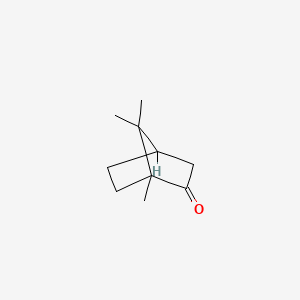 |
0.295 | ||
| ENC002322 |  |
0.339 | D0Q4XQ |  |
0.227 | ||
| ENC000146 | 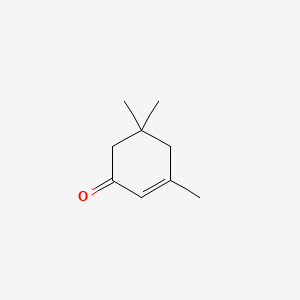 |
0.317 | D0V8HA | 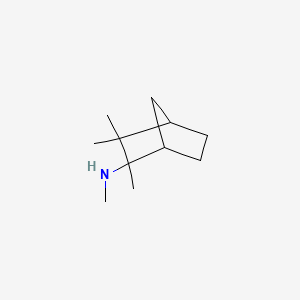 |
0.224 | ||
| ENC000481 |  |
0.295 | D0U4VT |  |
0.205 | ||
| ENC001193 |  |
0.283 | D0Q6NZ | 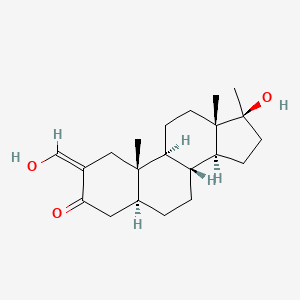 |
0.175 | ||
| ENC002418 |  |
0.278 | D0Z1XD | 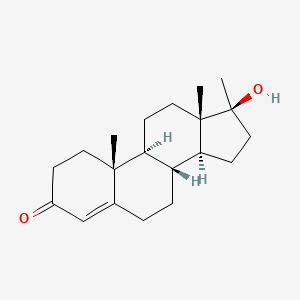 |
0.171 | ||
| ENC002262 |  |
0.268 | D0U3GL | 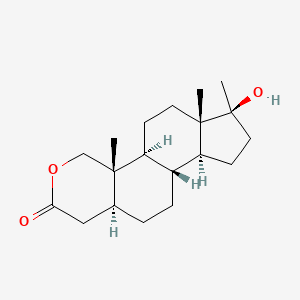 |
0.171 | ||
| ENC001370 |  |
0.260 | D05OQJ |  |
0.170 | ||
| ENC002058 |  |
0.259 | D0N0RU |  |
0.167 | ||
| ENC005896 |  |
0.259 | D09JBP |  |
0.167 | ||