NPs Basic Information

|
Name |
4-Carvomenthenol
|
| Molecular Formula | C10H18O | |
| IUPAC Name* |
4-methyl-1-propan-2-ylcyclohex-3-en-1-ol
|
|
| SMILES |
CC1=CCC(CC1)(C(C)C)O
|
|
| InChI |
InChI=1S/C10H18O/c1-8(2)10(11)6-4-9(3)5-7-10/h4,8,11H,5-7H2,1-3H3
|
|
| InChIKey |
WRYLYDPHFGVWKC-UHFFFAOYSA-N
|
|
| Synonyms |
4-Carvomenthenol; Terpinen-4-ol; 562-74-3; 4-Terpineol; p-Menth-1-en-4-ol; Terpinenol-4; 1-Terpinen-4-ol; 1-p-Menthen-4-ol; Terpene-4-ol; 1-Menthene-4-ol; TERPINENE-4-OL; 3-Cyclohexen-1-ol, 4-methyl-1-(1-methylethyl)-; 1-para-Menthen-4-ol; Terpinine-4-ol; (+-)-p-Menth-1-en-4-ol; rac Terpinen-4-ol; (+/-)-Terpinen-4-ol; 4-Methyl-1-(1-methylethyl)-3-cyclohexen-1-ol; 4-methyl-1-propan-2-ylcyclohex-3-en-1-ol; FEMA No. 2248; 4-Methyl-1-(propan-2-yl)cyclohex-3-en-1-ol; (+/-)-4-Terpineol; para-Menth-1-en-4-ol; 1-Methyl-4-isopropyl-1-cyclohexen-4-ol; 4-Methyl-1-isopropyl-3-cyclohexen-1-ol; Terpineol-4; Terpin-4-en-1-ol; (+/-)-1-Isopropyl-4-methyl-3-cyclohexen-1-ol; L65MV77ZG6; CHEBI:78884; 4-Terpinenol; 1-isopropyl-4-methylcyclohex-3-enol; MFCD00001562; NSC-147749; 1-isopropyl-4-methylcyclohex-3-en-1-ol; L-4-terpineneol; L-4-terpineol; L-terpinen-4-ol; Terpinenolu-4 [Czech]; Terpinenolu-4; dl-4-Terpineol; 4-Carvomenthenol (natural); NSC 147749; CCRIS 9067; EINECS 209-235-5; EINECS 248-910-9; BRN 1906603; UNII-L65MV77ZG6; Origanol; Terpinen 4-ol; alpha-terpinen-4-ol; alpha -Terpinen-4-ol; 1-Isopropyl-4-methyl-3-cyclohexen-1-ol, (R)-; 1-isopropyl-4-methyl-cyclohex-3-en-1-ol; DSSTox_CID_24824; DSSTox_RID_80505; (1)-1-(Isopropyl)-4-methylcyclohex-3-en-1-ol; 4-TERPINEOL [INCI]; DSSTox_GSID_44824; SCHEMBL22344; 4-06-00-00250 (Beilstein Handbook Reference); TERPINEN-4-OL [FCC]; (-)-p-Menth-1-en-4-ol; CHEMBL507795; 4-CARVOMENTHENOL [FHFI]; DTXSID4044824; FEMA 2248; HSDB 8264; (+/-)-p-Menth-1-en-4-ol; 4-TERPINEOL, (+/-)-; TERPINEN-4-OL,(+/-)-; Tox21_301785; AC1341; NSC147749; s6118; AKOS015903412; CS-W018032; DB12816; HY-W017316; SB44714; 4-Carvomenthenol, >=95%, FCC, FG; NCGC00256250-01; 1-Isopropyl-4-methyl-3-cyclohexen-1-ol; 4-Carvomenthenol, natural, >=95%, FG; AS-56462; CAS-562-74-3; SY012857; DB-066063; FT-0604405; FT-0604437; FT-0619472; M0319; T1993; (-)-1-Isopropyl-4-methyl-3-cyclohexen-1-ol; C17073; A918559; Q416114; (-)-4-Hydroxy-4-isopropyl-1-methyl-1-cyclohexene; 1-(ISOPROPYL)-4-METHYLCYCLOHEX-3-EN-1-OL; (+/-)-4-Hydroxy-4-isopropyl-1-methyl-1-cyclohexene; METHYL-1-(1-METHYLETHYL)-3-CYCLOHEXEN-1-OL; Terpinen 4-ol, primary pharmaceutical reference standard; 3-CYCLOHEXEN-1-OL; 4-METHYL-1-(1-METHYLETHYL)-
|
|
| CAS | 562-74-3 | |
| PubChem CID | 11230 | |
| ChEMBL ID | CHEMBL507795 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 154.25 | ALogp: | 2.2 |
| HBD: | 1 | HBA: | 1 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 20.2 | Aromatic Rings: | 1 |
| Heavy Atoms: | 11 | QED Weighted: | 0.575 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.217 | MDCK Permeability: | 0.00002010 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.002 |
| Human Intestinal Absorption (HIA): | 0.004 | 20% Bioavailability (F20%): | 0.547 |
| 30% Bioavailability (F30%): | 0.093 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.931 | Plasma Protein Binding (PPB): | 85.34% |
| Volume Distribution (VD): | 1.335 | Fu: | 18.45% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.274 | CYP1A2-substrate: | 0.258 |
| CYP2C19-inhibitor: | 0.112 | CYP2C19-substrate: | 0.846 |
| CYP2C9-inhibitor: | 0.058 | CYP2C9-substrate: | 0.704 |
| CYP2D6-inhibitor: | 0.025 | CYP2D6-substrate: | 0.278 |
| CYP3A4-inhibitor: | 0.04 | CYP3A4-substrate: | 0.261 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 14.345 | Half-life (T1/2): | 0.447 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.017 | Human Hepatotoxicity (H-HT): | 0.109 |
| Drug-inuced Liver Injury (DILI): | 0.05 | AMES Toxicity: | 0.007 |
| Rat Oral Acute Toxicity: | 0.025 | Maximum Recommended Daily Dose: | 0.025 |
| Skin Sensitization: | 0.456 | Carcinogencity: | 0.688 |
| Eye Corrosion: | 0.109 | Eye Irritation: | 0.966 |
| Respiratory Toxicity: | 0.024 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001637 |  |
1.000 | D06GIP |  |
0.208 | ||
| ENC001077 |  |
0.469 | D04CSZ |  |
0.208 | ||
| ENC003268 |  |
0.423 | D0P1UX |  |
0.197 | ||
| ENC005115 |  |
0.407 | D07QKN | 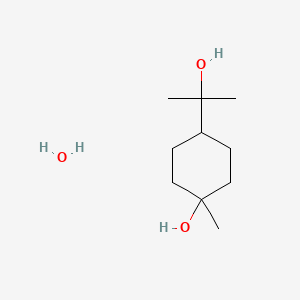 |
0.196 | ||
| ENC000520 |  |
0.366 | D08KVZ |  |
0.194 | ||
| ENC004025 |  |
0.358 | D0O3FG |  |
0.188 | ||
| ENC005252 |  |
0.348 | D0H1QY | 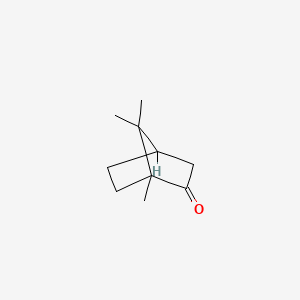 |
0.184 | ||
| ENC001824 |  |
0.346 | D01CKY | 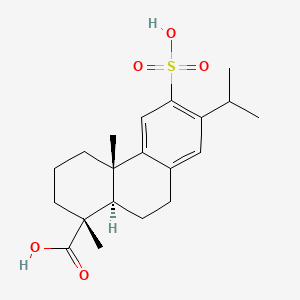 |
0.181 | ||
| ENC001813 |  |
0.346 | D0A2AJ |  |
0.179 | ||
| ENC000588 |  |
0.346 | D03KEK |  |
0.174 | ||