NPs Basic Information
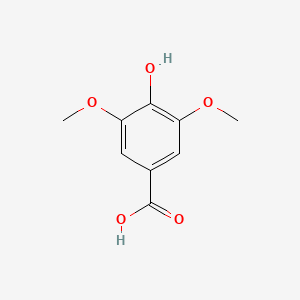
|
Name |
Syringic acid
|
| Molecular Formula | C9H10O5 | |
| IUPAC Name* |
4-hydroxy-3,5-dimethoxybenzoic acid
|
|
| SMILES |
COC1=CC(=CC(=C1O)OC)C(=O)O
|
|
| InChI |
InChI=1S/C9H10O5/c1-13-6-3-5(9(11)12)4-7(14-2)8(6)10/h3-4,10H,1-2H3,(H,11,12)
|
|
| InChIKey |
JMSVCTWVEWCHDZ-UHFFFAOYSA-N
|
|
| Synonyms |
SYRINGIC ACID; 530-57-4; 4-Hydroxy-3,5-dimethoxybenzoic acid; 3,5-Dimethoxy-4-hydroxybenzoic acid; Cedar acid; Gallic acid 3,5-dimethyl ether; Benzoic acid, 4-hydroxy-3,5-dimethoxy-; NSC 2129; 3,5-Dimethoxy-4-hydroxybenzyl acid; MFCD00002552; CHEMBL1414; CHEBI:68329; 4-Hydroxy-3,5-dimethoxy-benzoic acid; 4-Hydroxy-3,5-dimethoxybenzoicacid; NSC-2129; E390O181H5; EINECS 208-486-8; BRN 2115262; Syringlicacid; AI3-24376; UNII-E390O181H5; SYRA; SpecPlus_000485; Spectrum3_001866; Spectrum5_000963; Syringic acid, >=95%; bmse000607; bmse010206; SCHEMBL42751; BSPBio_003312; SYRINGIC ACID [INCI]; 4-10-00-01995 (Beilstein Handbook Reference); DivK1c_006581; 3,5-Dimethyl-4-hydroxybenzoate; DTXSID0060191; 3,5-dimethyl ether Gallic Acid; KBio1_001525; KBio3_002814; 3,5-Dimethoxy-4-hydroxybenzoate; NSC2129; HMS3885G17; Syringic acid, analytical standard; ZINC156386; HY-N0339; BBL012974; BDBM50187132; s3629; STL163855; 3,5-dimethoxy-4-hydroxy benzoic acid; AKOS000269664; 2,6-DIMETHOXY-4-CARBOXYPHENOL; AC-7975; CCG-214218; PS-8244; NCGC00178148-01; SY005479; 3,5-DIMETHOXY-4-HYDROXYBENZOICACID; DB-022071; CS-0008899; FT-0632317; G0014; A14823; EN300-117146; 530S574; Q408428; Q-100604; BRD-K51980294-001-01-9; F3157-0001; Z1255360342; 3,5-Dimethoxy-4-hydroxybenzoic acid, 4-Hydroxy-3,5-dimethoxy-benzoic acid
|
|
| CAS | 530-57-4 | |
| PubChem CID | 10742 | |
| ChEMBL ID | CHEMBL1414 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 198.17 | ALogp: | 1.0 |
| HBD: | 2 | HBA: | 5 |
| Rotatable Bonds: | 3 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 76.0 | Aromatic Rings: | 1 |
| Heavy Atoms: | 14 | QED Weighted: | 0.77 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.142 | MDCK Permeability: | 0.00001090 |
| Pgp-inhibitor: | 0.002 | Pgp-substrate: | 0.003 |
| Human Intestinal Absorption (HIA): | 0.028 | 20% Bioavailability (F20%): | 0.011 |
| 30% Bioavailability (F30%): | 0.067 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.457 | Plasma Protein Binding (PPB): | 50.89% |
| Volume Distribution (VD): | 0.459 | Fu: | 38.57% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.032 | CYP1A2-substrate: | 0.911 |
| CYP2C19-inhibitor: | 0.025 | CYP2C19-substrate: | 0.058 |
| CYP2C9-inhibitor: | 0.028 | CYP2C9-substrate: | 0.131 |
| CYP2D6-inhibitor: | 0.012 | CYP2D6-substrate: | 0.141 |
| CYP3A4-inhibitor: | 0.016 | CYP3A4-substrate: | 0.071 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.208 | Half-life (T1/2): | 0.946 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.034 | Human Hepatotoxicity (H-HT): | 0.154 |
| Drug-inuced Liver Injury (DILI): | 0.795 | AMES Toxicity: | 0.009 |
| Rat Oral Acute Toxicity: | 0.011 | Maximum Recommended Daily Dose: | 0.023 |
| Skin Sensitization: | 0.129 | Carcinogencity: | 0.034 |
| Eye Corrosion: | 0.292 | Eye Irritation: | 0.977 |
| Respiratory Toxicity: | 0.044 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC004830 | 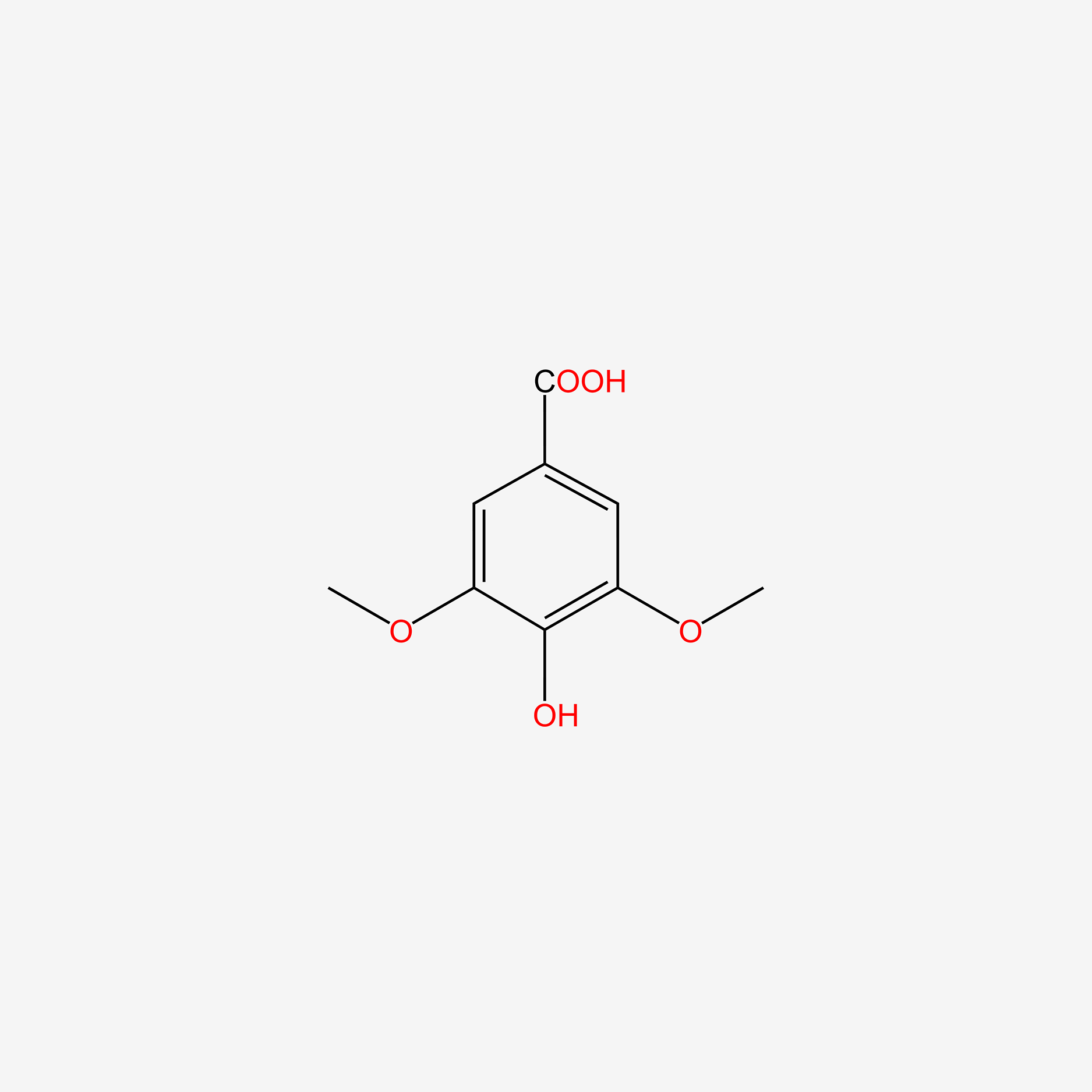 |
1.000 | D0E6OC |  |
0.316 | ||
| ENC000764 | 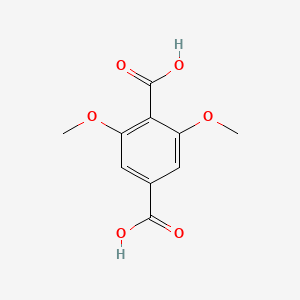 |
0.681 | D06GCK | 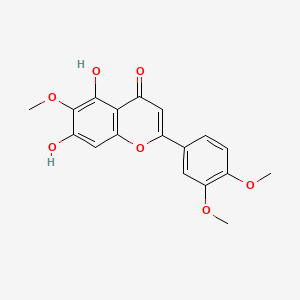 |
0.309 | ||
| ENC000304 |  |
0.600 | D02XJY |  |
0.303 | ||
| ENC004456 | 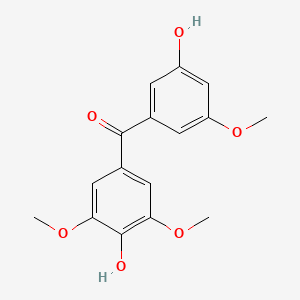 |
0.516 | D0A8FB |  |
0.292 | ||
| ENC000296 |  |
0.500 | D0E9CD | 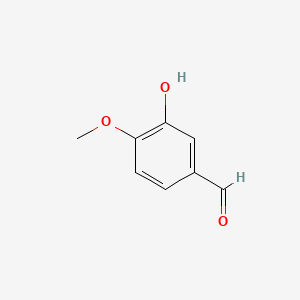 |
0.288 | ||
| ENC000478 |  |
0.469 | D09GYT | 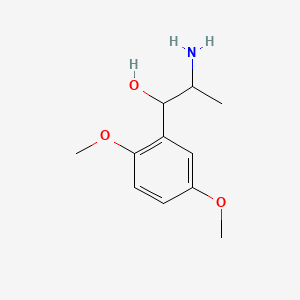 |
0.283 | ||
| ENC000712 |  |
0.469 | D0N1FS | 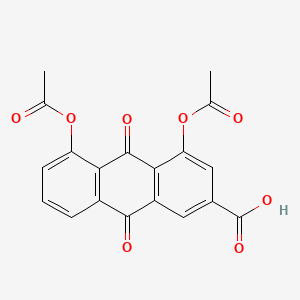 |
0.279 | ||
| ENC000168 |  |
0.457 | D06QKV | 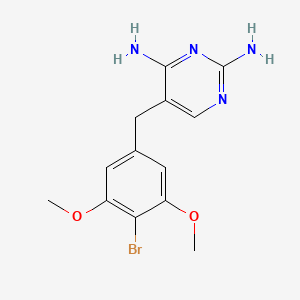 |
0.278 | ||
| ENC000671 |  |
0.451 | D0Q9ON |  |
0.275 | ||
| ENC000499 |  |
0.442 | D06TQZ |  |
0.274 | ||